- Anti-hestaminic & Respiratory Drugs (17)
- Anti-inflammatory Drugs (161) +-
- Baby & Mom (1146) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (47)
- Beauty (2395) +-
- Beauty > Skin Care > whitening (234)
- Chemotherapy & Immune Response (406) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (4)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (134)
- CIRCULATORY DISTURBANCE AGENTS (17)
- Diet & Fitness Products (192) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (153)
- Drugs affecting CNS >Anti- epileptic (64)
-
Medical Supplies (457)
+-
- Chemicals & Disinfectants (17)
- Dental Supplies (27)
- Devices & Instruments (8)
- Diabetic Supplies (95)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- Kindney Unit Supplies (14)
- Lab Supplies (1)
- Miscellaneous (22)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (4)
- Sanitary (5)
- Sterilization Supplies (0)
- Surgical Sutures (4)
- Syringes (2)
-
Medicines & Health (2246)
+-
- Allergy & Sinus (89)
- Children's Health Care (48)
- Cough, Cold & Flu (267)
- Digestive Health & Nausea (187)
- Ear, Nose & Throat Care (154)
- Eye Care (106)
- Feminine Care (288)
- Foot Care (3)
- Orthopaedic Appliances (0)
- Pain Relief & Management (188)
- Pill Organizer (0)
- Skin Treatments (644)
- Sleep & Snoring Aids (0)
- Support & Braces (6)
- Medicines & health > Gout releif (36)
- Natural & Organic Products (64) +-
- OTC > Analgesics > Anti-inflammatory Drugs (39)
-
Personal Care (2710)
+-
- Bath & Body (207)
- Deodorant & Anti-perspirants (160)
- Ear, Nose & Throat Care (147)
- Eye Care (112)
- Feminine Care (319)
- Foot Care (10)
- Hair Care (352)
- Home Tests & Monitorings (16)
- Incontinence (7)
- Lip Care (19)
- Massage & Relaxation (15)
- Natural & Organic Personal Care (7)
- Oral Care (74)
- Pregnancy & Fertility (54)
- Shaving & Grooming (53)
- Sun Care (52)
- Prescribtion drugs > cardiovascular system > Hypertention drugs (313) +-
-
Prescription Drugs (2571)
+-
- Analgesics (160)
- Cardiovascular System (308)
- Drugs Affecting Musculoskeletal System (57)
- Drugs Used In Infections (39)
- Ear & Nose Drugs (2)
- Endocrine System (133)
- Gastrointestinal Tract (195)
- Gastrointestinal Tract (180)
- GYNECOLOGY (2)
- Miscellaneous (5)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (14)
- NEUROLOGY (177)
- Nutrients & Blood Electrolytes (2)
- prescription drugs > cardiovascular system >Anti-hypertensive drugs (67)
- Prescription Drugs > Gastrointestinal Tract > Hepatology > Liver treatment (51)
- Respiratory System (108)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (21)
- Vaccines (1)
- Sexual Wellness (175) +-
- strong anti-emetic & adjuvent used with anti-neoplastic (0)
- Vitamins & Minerals Supplements (976) +-
Availability: Out Of Stock
Ex Tax: 480EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

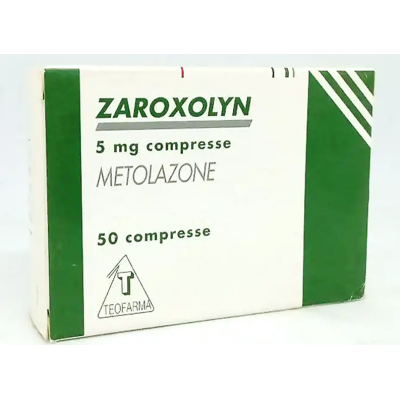
Zaroxolyn 5 mg Teofarma ( Metolazone ) 50 tablets
Zaroxolyn is indicated for the treatment of hypertension alone or, in the most severe forms, associated with other antihypertensive drugs. Zaroxolyn is also indicated as a diuretic in all cases of water-saline retention.
Zaroxolyn 5 mg tablets One tablet contains: Active ingredient: metolazone 5 mg. Zaroxolyn 10 mg tablets One tablet contains: Active ingredient: metolazone 10 mg.
Contraindications
Hypersensitivity to the active substance or to any of the excipients.
Severe liver and kidney failure.
Refractory hypokalaemia, symptomatic hyperuricaemia, Addison's disease.
dosage
Hypertension
: The recommended starting dose is 2.5 mg (equivalent to half a tablet) or 5 mg per day, in the morning.
Heart failure edema
: 5–10 mg once a day, in the morning;
Edema from kidney failure
: 5–20 mg once a day, in the morning.
After obtaining the desired therapeutic effect, it is normally advisable to reduce the dose of Zaroxolyn to the lowest levels of maintenance treatment (2.5 or 5 mg every other day).
The duration of the attack treatment with the highest posology can vary from a few days in edematous states, to 3–4 weeks in the treatment of hypertensive states.
Senior citizens
: lower starting doses should be used as they are more susceptible to side effects.
Patients with renal insufficiency
: the dosage should be changed according to renal function.
Pediatric age
: the use of the product is not recommended.
Warnings and Precautions
As with other diuretics, higher doses can produce a marked variation of potassium, uric acid, glucose and lipids in the plasma.
Electrolyte imbalances The product should be administered with caution in case of hypokalaemia, hyperkalaemia, hypercalcaemia and hypochloremic alkalosis.
Determinations of serum electrolytes (sodium, potassium, chlorine, calcium), in order to detect possible imbalances, must be carried out at regular intervals.
These checks are particularly important if the patient vomits excessively or is treated with parenteral fluids.
Also azotemia, uricemia and glucose must be periodically checked during diuretic therapy.
All patients treated with Zaroxolyn should be kept under observation in order to detect clinical signs of imbalances in the water-salt balance (hypokalaemia, hyponatraemia, hypochloremic alkalosis).
Some therapies, such as digitalis, can also be influenced by the effects of the diuretic on serum electrolytes (see 4.5).
The first signs of hydroelectrolytic imbalance, regardless of the cause, are: dry mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pain or cramps, muscle fatigue, hypotension, oliguria, tachycardia and gastrointestinal disorders (nausea, vomiting, etc.). ).
In case of hypokalaemia, a supplementary supply of potassium or the administration of a potassium-sparing drug is indicated.
Hypokalaemia can occur more often when diuretic therapy has been intense and prolonged, with concomitant steroid therapy or with ACTH, and with inadequate intake of salts.
Patients with hyperuricaemia and hyperglycaemia Caution should be exercised in these patients.
In subjects with latent diabetes, hyperglycaemia and glycosuria may occur.
In diabetic patients Zaroxolyn can interfere with antidiabetic therapy (see 4.5).
Hepatic or renal failure Diuretic-induced hypokalaemia can precipitate encephalopathy in liver failure.
Caution should be exercised when administering Zaroxolyn to patients with impaired renal function.
Since most of the drug is excreted through the kidneys, plasma levels of this drug can increase in such conditions.
Other situations Chloride deficiency and hypochloremic alkalosis may occur.
In patients with conspicuous edemas associated with heart failure or kidney failure, hyposaline syndrome may occur; a warm climate and a low salt diet can contribute to this.
Marked diuresis can cause acute hypotension.
Interactions
The combination of Zaroxolyn with the following drugs requires special precautions or a dosage adjustment: - Diuretics: increased risk of hypokalaemia; the administration of metolazone with furosemide determines a profuse diuresis that must be carefully followed.
- Other antihypertensives: increased hypotensive effect; adequate monitoring of blood pressure is necessary, especially in the initial phase, in order to promptly change the doses, if indicated.
- Opioid barbiturates and analgesics: increased hypotensive effect.
- Cyclosporine: increase in serum creatinine, if associated with metolazone.
Captopril: deterioration of renal function, which improves with the suspension of metolazone.
- Digital: increased toxicity with risk of serious arrhythmias, in particular in case of hypokalaemia - Corticosteroids and ACTH: increased risk of hypokalaemia and hydrosaline retention.
- Lithium: the elimination of lithium is reduced with an increase in its plasma concentrations and risk of toxicity.
- Neuromuscular blockers: increased neuromuscular blocking effect with respiratory depression up to apnea; therefore Zaroxolyn should be discontinued at least 3 days before surgery.
- Nonsteroidal anti-inflammatory drugs (NSAIDs): increased risk of NSAID nephrotoxicity.
NSAIDs can mitigate the antihypertensive effect of Zaroxolyn.
- Sympathomimetics: metolazone can decrease the response to norepinephrine, without however impeding its effectiveness as a pressure agent.
- Antidiabetics: reduction of the hypoglycaemic effect.
The need for an increase in the dose of hypoglycaemic agents should be considered.
- Anticoagulants: increased bleeding time has been observed with warfarin.
Zaroxolyn, like thiazide diuretics, can modify its hypoprothrombinemic response, hence the need to adjust the dose.
Alcohol can increase the hypotensive effect of metolazone.
Side effects
Headache, anorexia, vomiting, abdominal discomfort, muscle cramps and dizziness have occasionally been reported during treatment with Zaroxolyn.
Hyperuricemia and hyperazotemia have been reported mainly in patients with impaired renal function.
The undesirable effects reported with metazolone and listed below according to the organ class system are to be considered rare (<0.1%) or very rare (<0.01%): - Blood and lymphatic system disorders.
leukopenia, aplastic anemia, thrombocytopenia - Metabolism disorders and nutrition gout attacks - Nervous system disorders vertigo, somnolence, headache, paraesthesia, restlessness, insomnia, syncope - Eye disorders visual disturbances - Cardiac disorders palpitations, chest pains -Vascular disorders orthostatic hypotension, hypovolaemia, venous thrombosis - disorders of the gastrointestinal system constipation, dry mouth, nausea, vomiting, anorexia, diarrhea, flatulence, epigastric weight, pancreatitis - disorders of the hepatobiliary system intrahepatic cholestasis, hepatitis - skin and skin disorders subcutaneous tissue Exanthem, severe skin reactions - Disorders of the musculoskeletal and connective tissue cramps, muscle spasms - Diagnostic investigations hypokalaemia, hyponatraemia, hypochloremia, hypochloremic alkalosis, hypophosphataemia, glycosuria, increased blood sugar and creatinine, hyperuricemia.
- General disorders and alterations of the administration site hypersensitivity reactions: urticaria, purpura, necrotizing angiopathy.
Chills, asthenia.
When medium or severe side effects occur, the dose of Zaroxolyn should be reduced or treatment should be stopped.
Pregnancy and breastfeeding
Pregnancy Metolazone crosses the placental barrier and therefore its use in pregnancy is not recommended; There have been reports of jaundice and neonatal thrombocytopenia in babies born to mothers who took the drug.
Breastfeeding Metolazone passes into breast milk, therefore breastfeeding must be discontinued.
storage
Store away from light.
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: