- Anti-hestaminic & Respiratory Drugs (20)
- Anti-inflammatory Drugs (197) +-
- Baby & Mom (1346) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (52)
- Beauty (3129) +-
- Beauty > Skin Care > whitening (309)
- Chemotherapy & Immune Response (885) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (11)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (290)
- CIRCULATORY DISTURBANCE AGENTS (24)
- Diet & Fitness Products (284) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (191)
- HEMATOLOGY (39)
-
Medical Supplies (506)
+-
- Chemicals & Disinfectants (19)
- Dental Supplies (31)
- Devices & Instruments (11)
- Diabetic Supplies (121)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- KIDNEY UNIT SUPPLIES (21)
- Lab Supplies (3)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (2)
- Sanitary (5)
- Sterilization Supplies (1)
- Surgical Sutures (4)
- Syringes (3)
-
Medicines & Health (2727)
+-
- Allergy & Sinus (97)
- Children's Health Care (54)
- Cough, Cold & Flu (283)
- Digestive Health & Nausea (231)
- Ear, Nose & Throat Care (181)
- Eye Care (124)
- Feminine Care (323)
- Foot Care (12)
- Orthopaedic Appliances (1)
- Pain Relief & Management (244)
- Pill Organizer (2)
- Skin Treatments (863)
- Sleep & Snoring Aids (2)
- Support & Braces (8)
- Medicines & health > Gout releif (42)
- Natural & Organic Products (81) +-
- OTC > Analgesics > Anti-inflammatory Drugs (44)
-
Personal Care (3363)
+-
- Bath & Body (273)
- Deodorant & Anti-perspirants (191)
- Ear, Nose & Throat Care (177)
- Eye Care (131)
- Feminine Care (372)
- Foot Care (20)
- Hair Care (511)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (26)
- Massage & Relaxation (17)
- Natural & Organic Personal Care (7)
- Oral Care (91)
- Pregnancy & Fertility (64)
- Shaving & Grooming (75)
- Sun Care (80)
-
Prescription Drugs (2935)
+-
- Analgesics (184)
- Cardiovascular System (377)
- Drugs Affecting Musculoskeletal System (65)
- Drugs Used In Infections (56)
- Ear & Nose Drugs (2)
- Endocrine System (177)
- Gastrointestinal Tract (243)
- Gastrointestinal Tract > Hepatology > Liver treatment (60)
- GYNECOLOGY (2)
- Miscellaneous (11)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (47)
- NEUROLOGY (228)
- Nutrients & Blood Electrolytes (2)
- Respiratory System (154)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (115)
- Vaccines (1)
- Prescription drugs > Cardiovascular system > Anti-hypertension drugs (242)
- Sexual Wellness (304) +-
- Vitamins & Minerals Supplements (1230) +-
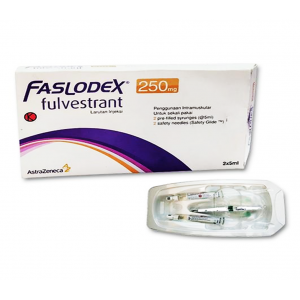
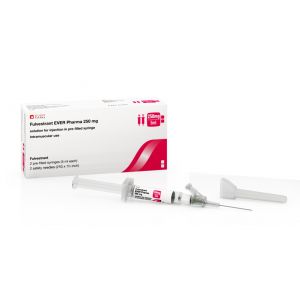
Ex Tax: 24,480EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

Important Safety Information and Indications
SUTENT can cause severe liver problems that can lead to death. Tell your healthcare provider right away if you develop any of the following signs and symptoms of liver problems during treatment with SUTENT:
- itching
- yellow eyes or skin
- dark urine
- pain or discomfort in the right upper stomach area
Your healthcare provider should do blood tests to check your liver function before you start taking and during treatment with SUTENT. Your healthcare provider may temporarily stop, reduce your dose, or permanently stop treatment with SUTENT if you develop liver problems.
SUTENT may cause serious side effects, including:
- Heart problems. Heart problems may include heart failure, heart attack and heart muscle problems (cardiomyopathy) that can lead to death. Tell your healthcare provider if you feel very tired, are short of breath, or have swollen feet and ankles.
- Abnormal heart rhythm changes. Changes in the electrical activity of your heart called QT prolongation can cause irregular heartbeats that can be life threatening. Your healthcare provider may do electrocardiograms and blood tests (electrolytes) to watch for these problems during your treatment with SUTENT. Tell your healthcare provider right away if:
- you feel faint or lightheaded, or you pass out
- you feel dizzy
- you feel your heart beat is irregular or fast
- High blood pressure. High blood pressure is common with SUTENT and may sometimes be severe. Follow your healthcare provider’s instructions about having your blood pressure checked regularly. Call your healthcare provider if your blood pressure is high or if you have any of the following signs or symptoms of high blood pressure:
- severe headache
- lightheadedness
- dizziness
- change in vision
Your healthcare provider may prescribe medicine for you to treat high blood pressure, if needed.
Bleeding problems:
Bleeding is common with SUTENT, but SUTENT can also cause severe bleeding problems that can lead to death. Your healthcare provider will monitor you for bleeding and do blood tests if needed. Call your healthcare provider right away if you have any of these symptoms or a serious bleeding problem during treatment with SUTENT, including:
- painful, swollen stomach (abdomen)
- vomiting blood
- black, sticky stools
- bloody urine
- headache
- change in your mental status
- coughing up blood
- Serious stomach and intestinal problems that can sometimes lead to death. Some people have had tears in their stomach or intestine (perforation) or have developed an abnormal opening between the stomach and intestine (fistula). Get medical help right away if you get stomach-area (abdominal) pain that does not go away or is severe during treatment with SUTENT.
- Tumor lysis syndrome (TLS). TLS is caused by the fast breakdown of cancer cells and may lead to death. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure, and sometimes death. Your healthcare provider may do blood tests to check you for TLS.
- Abnormal changes in the brain (reversible posterior leukoencephalopathy syndrome [RPLS]). RPLS can cause a collection of symptoms including headache, confusion, and vision loss. Some people who have taken SUTENT have developed RPLS that can lead to death.
- Thrombotic microangiopathy (TMA) including thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). TMA is a condition that involves injury to the smallest blood vessels and blood clots can happen while taking SUTENT. TMA is accompanied by a decrease in red cells and cells that are involved with clotting. TMA may harm your body’s organs such as the brain and kidneys, and can sometimes lead to death.
- Protein in your urine. Some people who have taken SUTENT have developed protein in their urine, and in some cases, kidney problems that can lead to death. Your healthcare provider will check you for this problem.
Serious skin and mouth reactions. Treatment with SUTENT has caused severe skin reactions that can lead to death, including:
- severe rash with blisters or peeling of the skin.
- painful sores or ulcers on the skin, lips, or inside the mouth.
- tissue damage (necrotizing fasciitis).
If you have any signs or symptoms of severe skin reactions, stop taking SUTENT and call your healthcare provider or get medical help right away.
- Thyroid problems. Your healthcare provider may do tests to check your thyroid function during SUTENT treatment. Tell your healthcare provider if you have any of the following signs and symptoms during your treatment with SUTENT:
- tiredness that gets worse and does not go away
- loss of appetite
- problems with heat
- feeling nervous or agitated, tremors
- sweating
- nausea or vomiting
- diarrhea
- fast heart rate
- weight gain or weight loss
- feeling depressed
- irregular menstrual periods or no menstrual periods
- headache
- hair loss
- Low blood sugar (hypoglycemia). Low blood sugar can happen with SUTENT, and may cause you to become unconscious, or you may need to be hospitalized. Low blood sugar with SUTENT may be worse in people who have diabetes and take antidiabetic medicines. Your healthcare provider should check your blood sugar levels regularly during treatment with SUTENT and may need to adjust the dose of your antidiabetic medicines. Call your healthcare provider right away if you have any of the following signs or symptoms of low blood sugar during your treatment with SUTENT:
- headache
- drowsiness
- weakness
- dizziness
- confusion
- irritability
- hunger
- fast heartbeat
- sweating
- feeling jittery
- Jawbone problems ( osteonecrosis ). Severe jawbone problems have happened in some people who take SUTENT. Certain risk factors such as taking a bisphosphonate medicine or having dental disease may increase your risk of getting osteonecrosis. Your healthcare provider may tell you to see your dentist before you start taking SUTENT. Your healthcare provider may tell you to avoid dental procedures, if possible, during your treatment with SUTENT, especially if you are receiving a bisphosphonate medicine into a vein (intravenous). Tell your healthcare provider if you plan to have any dental procedures before or during treatment with SUTENT.
- You should stop taking SUTENT at least 3 weeks before planned dental procedures.
- Your healthcare provider should tell you when you may start taking SUTENT again after dental procedures.
- Wound healing problems. Wound healing problems have happened in some people who take SUTENT. Tell your healthcare provider if you plan to have any surgery before or during treatment with SUTENT.
- You should stop taking SUTENT at least 3 weeks before planned surgery.
- Your healthcare provider should tell you when you may start taking SUTENT again after surgery.
- Fetal harm. SUTENT can harm your unborn baby. Tell your healthcare provider if you are pregnant or plan to become pregnant. You and your partner should use effective birth control (contraception) during and after treatment with SUTENT.
- Females should continue to use birth control for at least 4 weeks after their last dose of SUTENT.
- Males should continue to use birth control for at least 7 weeks after their last dose of SUTENT.
Your healthcare provider may temporarily stop, reduce your dose, or permanently stop treatment with SUTENT if you develop serious side effects.
Before taking SUTENT tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems
- have high blood pressure
- have thyroid problems
- have a history of low blood sugar or diabetes
- have kidney function problems (other than cancer)
- have liver problems
- have any bleeding problem
- plan to have surgery or have had recent surgery. You should stop taking SUTENT at least 3 weeks before planned surgery.
- have seizures
- have or have had pain in the mouth, teeth, or jaw, swelling or sores inside the mouth, numbness or a feeling of heaviness in the jaw, or loosening of a tooth
- are breastfeeding or plan to breastfeed. Do not breastfeed during treatment with SUTENT and for at least 4 weeks (1 month) after the last dose.
- are pregnant or plan to become pregnant. SUTENT can harm your unborn baby.
Females who are able to become pregnant:
- Your healthcare provider should do a pregnancy test before you start treatment with SUTENT.
- You should use effective birth control (contraception) during treatment and for at least 4 weeks after your last dose of SUTENT.
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with SUTENT.
Males with female partners who are able to become pregnant should use effective birth control (contraception) during treatment and for 7 weeks after your last dose of SUTENT.
SUTENT may cause fertility problems in males and females. Tell your healthcare provider if this is a concern for you.
Tell all of your healthcare providers and dentists that you are taking SUTENT. They should talk to the healthcare provider who prescribed SUTENT for you, before you have any surgery, or medical or dental procedure.
Tell your healthcare provider about all the medicines you take, including prescription medicines and over-the-counter medicines, vitamins, and herbal supplements. Using SUTENT with certain other medicines can cause serious side effects.
You may have an increased risk of severe jawbone problems (osteonecrosis) if you take SUTENT and a bisphosphonate medicine. Especially tell your healthcare provider if you are taking or have taken an osteoporosis medicine.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
Do not drink grapefruit juice or eat grapefruit. They may cause you to have too much SUTENT in your body.
Do not take St. John’s Wort. It may cause you to have too little SUTENT in your body.
Common side effects of SUTENT include:
- tiredness
- weakness
- diarrhea
- pain, swelling, or sores inside of your mouth
- nausea
- loss of appetite
- indigestion
- vomiting
- stomach-area (abdominal) pain
- blisters or rash on the palms of your hands and soles of your feet
- high blood pressure
- taste changes
- low platelet counts
The medicine in SUTENT is yellow, and it may make your skin look yellow. Your skin and hair may get lighter in color. SUTENT may also cause other skin problems including: dryness, thickness, or cracking of the skin.
These are not all of the possible side effects of SUTENT.
Call your doctor for medical advice about side effects. You are encouraged to report adverse events related to Pfizer products by calling 1-800-438-1985. If you prefer, you may contact the US Food and Drug Administration (FDA) directly.
Visit www.fda.gov/MedWatch or call 1-800-FDA-1088.
INDICATIONS
SUTENT is a prescription medicine used to treat:
- a rare cancer of the stomach, bowel, or esophagus called gastrointestinal stromal tumor (GIST) and when:
- you have taken the medicine imatinib mesylate (Gleevec®) and it did not stop the cancer from growing or
- you cannot take imatinib mesylate (Gleevec®).
- advanced kidney cancer (advanced renal cell carcinoma or RCC).
- adults with kidney cancer that has not spread (localized) and who are at high risk of RCC coming back again after having kidney surgery.
- a type of pancreatic cancer called pancreatic neuroendocrine tumors (pNET) that has progressed and cannot be treated with surgery.
It is not known if SUTENT is safe and effective in children.
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: