- Anti-hestaminic & Respiratory Drugs (20)
- Anti-inflammatory Drugs (197) +-
- Baby & Mom (1346) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (52)
- Beauty (3129) +-
- Beauty > Skin Care > whitening (309)
- Chemotherapy & Immune Response (885) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (11)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (290)
- CIRCULATORY DISTURBANCE AGENTS (24)
- Diet & Fitness Products (284) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (191)
- HEMATOLOGY (39)
-
Medical Supplies (506)
+-
- Chemicals & Disinfectants (19)
- Dental Supplies (31)
- Devices & Instruments (11)
- Diabetic Supplies (121)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- KIDNEY UNIT SUPPLIES (21)
- Lab Supplies (3)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (2)
- Sanitary (5)
- Sterilization Supplies (1)
- Surgical Sutures (4)
- Syringes (3)
-
Medicines & Health (2727)
+-
- Allergy & Sinus (97)
- Children's Health Care (54)
- Cough, Cold & Flu (283)
- Digestive Health & Nausea (231)
- Ear, Nose & Throat Care (181)
- Eye Care (124)
- Feminine Care (323)
- Foot Care (12)
- Orthopaedic Appliances (1)
- Pain Relief & Management (244)
- Pill Organizer (2)
- Skin Treatments (863)
- Sleep & Snoring Aids (2)
- Support & Braces (8)
- Medicines & health > Gout releif (42)
- Natural & Organic Products (81) +-
- OTC > Analgesics > Anti-inflammatory Drugs (44)
-
Personal Care (3363)
+-
- Bath & Body (273)
- Deodorant & Anti-perspirants (191)
- Ear, Nose & Throat Care (177)
- Eye Care (131)
- Feminine Care (372)
- Foot Care (20)
- Hair Care (511)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (26)
- Massage & Relaxation (17)
- Natural & Organic Personal Care (7)
- Oral Care (91)
- Pregnancy & Fertility (64)
- Shaving & Grooming (75)
- Sun Care (80)
-
Prescription Drugs (2935)
+-
- Analgesics (184)
- Cardiovascular System (377)
- Drugs Affecting Musculoskeletal System (65)
- Drugs Used In Infections (56)
- Ear & Nose Drugs (2)
- Endocrine System (177)
- Gastrointestinal Tract (243)
- Gastrointestinal Tract > Hepatology > Liver treatment (60)
- GYNECOLOGY (2)
- Miscellaneous (11)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (47)
- NEUROLOGY (228)
- Nutrients & Blood Electrolytes (2)
- Respiratory System (154)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (115)
- Vaccines (1)
- Prescription drugs > Cardiovascular system > Anti-hypertension drugs (242)
- Sexual Wellness (304) +-
- Vitamins & Minerals Supplements (1232) +-
Ex Tax: 65EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

INFLAMOFEN 3% TOPICAL CUTANEOUS FOAM ( DICLOFENAC SODIUM 32.22 MG/GM ) 50 GM
INFLAMOFEN 3%
Topical cutaneous foam
1. Name of the medicinal product
- Inflamofen 3% .
2. Qualitative and quantitative composition
- 100 g of cutaneous foam contain:
- Diclofenac 3 g.
- For the full list of excipients, see section 6.1.
3. Pharmaceutical form
- Topical cutaneous foam.
- White to yellowish white foam.
4. Clinical information
4.1. Therapeutic indications
- Local treatment of painful and inflammatory
conditions of a rheumatic or traumatic conditions of
the joints, muscles, tendons and ligaments.
4.2. Dosage and method of administration
- Adults and adolescents from 14 years of age:
Apply Inflamofen cutaneous foam 1-3 times a day.
For each application, spray on the palm of the hand.
depending on the size of the area to be treated, a
circular mass of foam of 3-5 centimeters in diameter
(equal to about 0.75-1.5 grams in weight), massaging
gently until complete absorption. In case of
iontophoresis treatment, the product must be applied
to the negative pole. INFLAMOFEN cutaneous foam
can also be used in combination with ultrasound
therapy.
- After application, ask the patient to wash their hands,
to avoid subjecting them to the treatment.
- Warning: the product should only be used for short
periods of treatment.
- If the product is needed for more than 7 days to
relieve pain or if symptoms worsen, the doctor should
re-evaluate the situation (see section 4.4).
- Children under the age of 14 years: insufficient data
are available on the efficacy and safety in children
and adolescents under the age of 14 years (see also
section 4.3 Contraindications).
- Therefore, the use of Inflamofen cutaneous foam is
contraindicated in children under 14 years of age.
- Elderly: The usual adult dosage may be used.
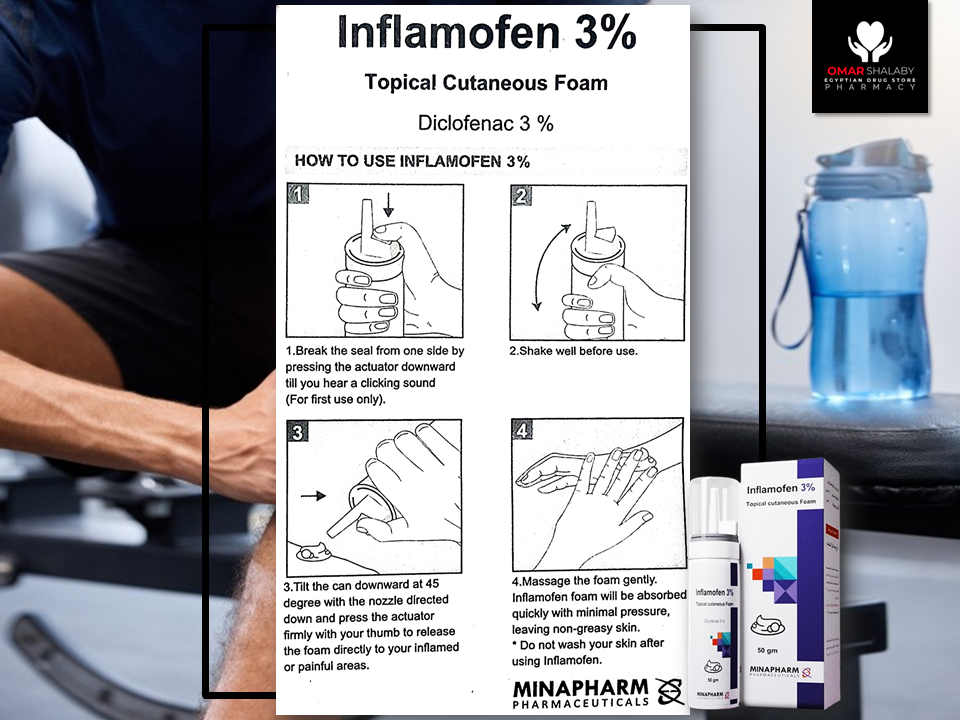
- How to use; Shake before use. With the can upside
down, dispense the desired amount by pressing the
appropriate dispenser. For cutaneous use only.
4.3. Contraindications
- Hypersensitivity to the active substance or to any of
the excipients listed in section 6.1.
- Patients with a history of asthma, angioedema,
urticarla or acute rhinitis after taking acetylsalicylic
acid or other non-steroidal anti-inflammatory drugs
(NSAIDs).
- Damaged skin, regardless of the type of lesion:
exudative dermatitis, eczema, infected lesion, bums
or wounds (see section 4.4).
- Third trimester of pregnancy (see section 4.6).
- Children and adolescents under the age of 14.
4.4. Speclal warnings and procautions for Usk
. The possibility of syslemic adverse events with the
application of Inflamofen cannot be excluded if the
preparation is used on large skin areas and for a
Inflamofen should only be applied to intact, healthy
skin, and not to cutaneous wounds or open lesions. It
should not be allowed to come into contact with eyes
or mucous membranes and should not be ingested.
. Treatment should be discontinued if skin rash
develops after application of the product.
. Inflamolen can be used wilh non-occlusive dressings.
but must not be used with an occlusive dressing that
does not allow air to pass.
- The concomitant use of systemic anti-inflammatory
drugs is not recommended in elderly and / or patients
with gastric problems. Asthmatic patients, with chronic
cbstructive diseases of the bronchi, allergic rhinitis
or inflammation of the nasal mucosa (nasal polyp).
react, with asthmatic attacks, local inflammation of
the skin, mucosa (Quincke's edema) or urticaria, to
the antirheumatic treatment carried out with NSAIDs.
more often than other patients.
The administration of Inflamofen should be
discontinued in women who have fertility problems
or who are undergoing fertility investigations. The
use of Inflamofen, especially if prolonged, can give
rise to local sensitization phenomena, which require
the interruption of the treatment and the adoption
of adequate therapeutic measures. To reduce any
phenomena of photosensitivity, patients should be
wamed not to expose themselves to direct sunlight or
the light of sun lamps during use. In case of allergic
reactions or adverse reactions of greater importance.
it is necessary to discontinue treatment with
Inflamofen and institute adequate therapy.
The use of the medicine in combination with other
medicines containing diclofenac can give rise to skin
reactions with severe evolution (Stevens-Johnson
syndrome, Lyell's syndrome).
- Inflamofen contains 0.25 g benzyl alcohol (equivalent
to 0.5%w/w)
- Benzyl alcohol can cause allergic reactions and mild
local irritation.
- Inflamofen contains an aroma which in tum contains
limonene.
- Limonene can cause allergic reactions.
- Elevation of one or more liver tests may occur during
therapy with diclofenac. Diclofenac gel should be
discontinued immediately if abnormal liver tests
persist or worsen.
- In postmarketing reports, cases of drug-induced
hepatotoxicity have been reported in the first month,
and in some cases, the first 2 months of therapy.
but can occur at any time during treatment with
diclofenac
Post marketing surveillance has reported cases of
severe hepatic reactions, including liver necrosis,
jaundice, fulfillment hepatitis with and without
jaundice, and liver failure Sore of these reported
cases resulted in fatalities or liver transplantation.
Physicians should measure transaminases
periodically in patients receiving long-torm therapy
with diclofenac, because severe hopalotoxicity
may develop without a prodrome of distinguishing
symploms.
4.5. Interactions with other medicinos and othor
forms of interaction
The systemic absorption of diclofenac following
topical application is very low.
- In high-dose and long-term treatments, the possibility
of competition between absorbed diclofenac and
other drugs with high plasma protein binding should
be taken into account.
4.6. Fertility, pregnancy and breastfeeding
Pregnancy
- The systemic concentration of diclofenac compared
with oral formulations is lower after topical
administration. Referring to experience with NSAID
treatment for systemic administration, the following is
recommended:
Inhibition of prostaglandin synthesis can negatively
affect pregnancy and / or embryo / fetal development.
- Results of epidemiological studies suggest
an increased risk of miscarriage and cardiac
malformation and gastroschisis after the use of a
prostaglandin synthesis inhibitor in the early stages
of pregnancy.
- The absolute risk of heart malformations increased
from less than 1%, up to about 1.5%. It was believed
that the risk increases with the dose and duration of
therapy. In animals, administration of prostaglandin
synthesis Inhibitors has been shown to cause an
increase in pre- and post-implantation loss and
embryo-fetal mortality.
- In addition, an increased incidence of various
malformations, including cardiovascular, has been
reported in animals given prostaglandin synthesis
inhibitors during the organogenetic period. During the
first and second trimester of pregnancy, diclofenac
should not be administered except in strictly
necessary cases. If diclofenac is used by a woman
attempting to conceive, or during the first and second
trimester of pregnancy, the dose should be kept as
low as possible and the duration of treatment as short
as possible.
- During the third trimester of pregnancy, all
prostaglandin synthesis inhibitors can expose the
fetus to:
- Cardiopulmonary toxicity (with premature closure of
the arterial duct and pulmonary hypertension):
- Renal dysfunction, which can progress to renal failure
with oligo-hydroamnios;
- the mother and the newborn, at the end of pregnancy
due to:
- Possible prolongation of bleeding time, and antiplate-
let effect which may occur even at very low doses;
. inhibition of uterine contractions resulting in delayed
or prolonged labor.
- Consequently, diclofenac is contraindicated during the
third trimester of pregnancy.
Breastfeeding
. Like other NSAIDs, diclofenac passes into breast
milk in small amounts. Howover, at therapeutic doses
of Inflamofen no effects on the suckling child are
anticipated. Due to the lack of controlled studies in
breastfeeding women, the product should only be
used during breastfeeding if the expected benefit to
the mother outweighs the risk to the baby.
. In this circumstance. Inflamofen should not be applied
to the breasts of nursing mothers, or elsewhere on
large areas of skin or for an extended period of time
(see section 4.4).
Fertility
. There are no data on the effects of diclofenac for
topical use on fertility
4.7. Effects on the ability to drive and use
machines
. Inflamofen has no or negligible influence on the
ability to drive or use machines.
4.8. Side effects
. Adverse reactions (Table 1) are listed by frequency.
starting from the most frquent adverse reaction, as
follows; common (2 1/100 to <1/10), uncommon (2
1/1000 to <1/100), rare (2 1 / 10,000 to <1 / 1,000).
very rare (<1 / 10.000); Not known: cannot be
estimated from the available data.
table 1.
| Disorders of the immune system - Very rare: Hypersensitivity (including urticaria). angioneurotic edema |
| Infections and infestations - Very rare: Rash with pustules. |
| Respiratory, thoracic and mediastinal disorders - Very rare: Asthma |
| Skin and subcutaneous tissue disorders - Common: Rash, eczema, erythena, dermalitis (including contact dermatitis), pruritus - Rare: Bullous dermatitis, burning. - Very rare: Photosensitivity reaction. |
Reporting of suspected adverse reactions:
- Reporting suspected adverse reactions after
authorization of the medicinal product is important.
It allows continued monitoring of the benefit/
risk balance of the medicinal product. Healthcare
professionals are asked to report any suspected
adverse reactions via: pv.followup@edaegypt.gov.eg
4.9. Ovordose
- There have been reports of overdose with Inflamofen.
but no systemic adverse effects that may be caused
by overdose with oral NSAIDs (e.g. vomiting.
diarrhea, dizziness, tinnitus, gastrointestinal
hemorrhage, convulsions) have been reported.
- However, undesirable effects similar to those seen
after an overdose of diclofenac tablets may be
expected if topical diclofenac is inadvertently ingested
(1 pressurized container of 50 g foam contains
approximately 1.611 g diclofenac sodium). In case of
accidental ingestion, which gives rise to significant
systemic side effects, the general therapeutic
measures normally adopted to treat poisoning with
non-steroidal anti-inflammatory drugs must be taken.
- Additional treatment modalities should take into
account the recommendations of the poison control
center, where available.
5. Pharmacological properties
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: non-steroidal anti-
inflammatory drugs for topical use.
- Diclofenac - active ingredient of the Inflamofen
specialty - exerts a marked anti-inflammatory-
analgesic action in painful states of a rheumatic and /
or traumatic conditions.
Mechanism of action:
- The action of diclofenac is expressed in part
through the competitive and irreversible inhibition
of the biosynthesis of prostaglandins and in part
through the inhibition of lysosomal enzymes. The
pharmacodynamics of controlled effects in rats
with damaged skin showed an increase in the
pain reaction threshold and a reduction in edema
The analgesic effect of diclofonac administered
topically was also confirmed in healthy volunteers in
experimental pain provocation models.
5.2. Pharmacokinetic properties
a) General characteristics of the active ingredient
- Pharmacokinetic data in healthy volunteers show
that, following the application of Inflamofen, the
active ingredient is absorbed more rapidly compared
to a topical gel containing diclofenac used as a
comparison
- The plasma levels achieved with both products are
similar (less than 10 ng / ml) and are approximately
100 times lower than those achieved by administering
equal doses of oral diclofenac. These results are
expected for topical action products and are based on
the absence of systemic effects of the product. Even
after repeated administration of Inflamofen for 6 days
there was no evidence of accumulation of diclofenac
in the plasma
b) Characteristics of particular interest to the patient
- The application of Inflamofen satisfies the need
for an effective and safe local treatment suitable to
avoid concomitant systemic administration of anti-
inflammatory drugs not recommended in elderly and /
or patients with gastric disturbances.
c) Characteristics of particular interest to the patient
- The application of Inflamofen satisfies the need
for an effective and safe local treatment suitable to
avoid concomitant systemic administration of anti-
inflammatory drugs not recommended in elderly and /
or patients with gastric disturbances.
6. Pharmaceutical information
6.1. List of excipients
- Caprylocaproyl macrogol glycerides, Soya lecithin,
benzyl alcohol, potassium sorbate, polysorbate
80, dl-o-tocopheryl acetate, disodium phosphate
dodecahydrate, mint fragrance, eucalyptus fragrance.
purified water.
6.2. Incompatibility
- None
6.3. Shelf life
- 2 years
6.4. Special precautions for storage
- Store at temperature not exceeding 30°C, protect
from light.
6.5. Nature and contents of the container
- Carton box contains aluminum pressurized container
with release valve, stem (polyoxymethylene).
mounting cup (Aluminum Alloy), spring (stainless
stoel, (HDPE) plastic housing, polypropylene actuator
and transparent polypropylene plastic cap contains 50
gm solution and inner leaflet
6.6. Special precautions for disposal and handling
- Do not place or burn even after use.
Manufacturer:
Minapharm company for Pharmaceuticals & Chemical Industries (2)
&
License holder:
- Minapharm company for Pharmaceuticals & Chemical Industries


Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: