- Anti-hestaminic & Respiratory Drugs (20)
- Anti-inflammatory Drugs (192) +-
- Baby & Mom (1326) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (52)
- Beauty (3052) +-
- Beauty > Skin Care > whitening (307)
- Chemotherapy & Immune Response (883) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (11)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (289)
- CIRCULATORY DISTURBANCE AGENTS (24)
- Diet & Fitness Products (284) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (192)
- HEMATOLOGY (39)
-
Medical Supplies (503)
+-
- Chemicals & Disinfectants (19)
- Dental Supplies (31)
- Devices & Instruments (10)
- Diabetic Supplies (121)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- KIDNEY UNIT SUPPLIES (21)
- Lab Supplies (3)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (2)
- Sanitary (5)
- Sterilization Supplies (0)
- Surgical Sutures (4)
- Syringes (3)
-
Medicines & Health (2692)
+-
- Allergy & Sinus (95)
- Children's Health Care (54)
- Cough, Cold & Flu (277)
- Digestive Health & Nausea (230)
- Ear, Nose & Throat Care (179)
- Eye Care (124)
- Feminine Care (325)
- Foot Care (9)
- Orthopaedic Appliances (1)
- Pain Relief & Management (241)
- Pill Organizer (2)
- Skin Treatments (843)
- Sleep & Snoring Aids (2)
- Support & Braces (8)
- Medicines & health > Gout releif (42)
- Natural & Organic Products (81) +-
- OTC > Analgesics > Anti-inflammatory Drugs (44)
-
Personal Care (3279)
+-
- Bath & Body (271)
- Deodorant & Anti-perspirants (191)
- Ear, Nose & Throat Care (175)
- Eye Care (131)
- Feminine Care (374)
- Foot Care (17)
- Hair Care (472)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (22)
- Massage & Relaxation (17)
- Natural & Organic Personal Care (7)
- Oral Care (91)
- Pregnancy & Fertility (65)
- Shaving & Grooming (65)
- Sun Care (80)
-
Prescription Drugs (2905)
+-
- Analgesics (181)
- Cardiovascular System (374)
- Drugs Affecting Musculoskeletal System (65)
- Drugs Used In Infections (56)
- Ear & Nose Drugs (2)
- Endocrine System (176)
- Gastrointestinal Tract (241)
- Gastrointestinal Tract > Hepatology > Liver treatment (59)
- GYNECOLOGY (2)
- Miscellaneous (11)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (46)
- NEUROLOGY (225)
- Nutrients & Blood Electrolytes (2)
- Respiratory System (154)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (100)
- Vaccines (1)
- Prescription drugs > Cardiovascular system > Anti-hypertension drugs (242)
- Sexual Wellness (301) +-
- Vitamins & Minerals Supplements (1212) +-
Availability: In Stock
Ex Tax: 2,658EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

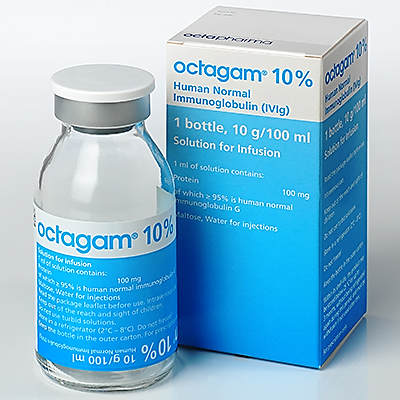
octagam 10%
PACKAGE LEAFLET: INFORMATION FOR THE USER
OCTAGAM 10%, solution for infusion
Human Normal Immunoglobulin (IVIg)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Octagam 10% is and what it is used for
2. What you need to know before you use Octagam 10%
3. How to use Octagam 10%
4. Possible side effects
5. How to store Octagam 10%
6. Contents of the pack and other information
1 What Octagam 10% is and what it is used for
What Octagam 10% is
Octagam 10% is a human normal immunoglobulin (IgG) solution (i.e. solution of human antibodies) for intravenous administration (i.e. infusion into a vein). Immunoglobulins are normal constituents of the human body and support the immune defence of your body. Octagam 10% contains all IgG activities which are present in the normal population. Adequate doses of this medicinal product may restore abnormally low IgG levels to the normal range.
Octagam 10% has a broad spectrum of antibodies against various infectious agents.
What Octagam 10% is used for
Octagam 10% is used as replacement therapy in children, adolescents (0-18 years) and adults in different groups of patients:
- Patients with inborn deficiency of antibodies (primary immunodeficiency syndromes, such as congenital agammaglobulinaemia and hypogammaglobulinaemia, common variable immunodeficiency, severe combined immunodeficiencies)
- Patients with an acquired deficiency of antibodies (secondary immunodeficiency) due to specific diseases and/or treatments and experiencing severe or recurrent infections
Octagam 10% can be further used in the treatment of the following autoimmune disorders (immunomodulation):
- in patients with immune thrombocytopenia (ITP), a condition where the platelets get destroyed and are therefore reduced in number, and who have a high risk of bleeding or need to correct the platelet count prior to surgery.
- in patients with Kawasaki disease, a condition that leads to inflammation of various organs.
- in patients with Guillain Barré syndrome, a condition that leads to inflammation of certain parts of the nervous system.
- in patients with chronic inflammatory demyelinating polyneuropathy (CIDP), a disease that leads to chronic inflammation of the peripheral parts of the nervous system which causes muscle weakness and/or numbness mainly in the legs and arms.
- in patients with multifocal motor neuropathy (MMN), a condition that is characterized by slow progressive asymmetrical weakness of limbs without sensory loss.
2 What you need to know before you use Octagam 10%
Do not use Octagam 10%:
- if you are allergic to human immunoglobulin or any of the other ingredients contained in Octagam 10% (listed in section 6).
- if you have a deficiency of immunoglobulin A (IgA deficiency) and if you have developed antibodies against immunoglobulins of the type IgA.
Warnings and precautions
Talk to your doctor or pharmacist before using Octagam 10%.
It is strongly recommended that every time you receive a dose of Octagam 10% the name and batch number of the product are recorded in order to maintain a record of the batches used.
Certain adverse reactions may occur more frequently:
- in case of high rate of infusion
- when you receive Octagam 10% for the first time or, in rare cases, when there has been a long interval since the previous infusion.
- when you have an untreated infection or an underlying chronic inflammation
In the case of an adverse reaction, either the rate of administration must be reduced or the infusion must be stopped. The treatment of the adverse event required will depend on the nature and severity of the side effect.
Circumstances and conditions increasing the risk of having side effects
- Thromboembolic events such as heart attack, stroke, and obstructions of a deep vein for example in the calves or of a blood vessel in the lung may occur very rarely after administration of Octagam 10%. These types of events occur more commonly, although very rarely, in patients with risk factors, such as obesity, advanced age, high blood pressure, diabetes, previous occurrences of such events, prolonged periods of immobilisations, and intake of certain hormones (e.g. the pill). Ensure a balanced fluid intake; moreover Octagam 10% should be administered as slowly as possible.
- If you had kidney problems in the past or if you have certain risk factors like diabetes, overweight, or age over 65, Octagam 10% should be administered as slowly as possible because cases of acute kidney failure have been reported in patients, although very rarely, with such risk factors. Tell your doctor, even when any of the above-mentioned circumstances had happened to you in the past.
- Patients with blood group A, B or AB as well as patients with certain inflammatory conditions have a higher risk of red blood cells being destroyed by the administered immunoglobulins (called haemolysis).
When may slowing or stopping the infusion be required?
- Strong headaches and neck stiffness may rarely occur several hours to 2 days following Octagam 10% treatment.
- Allergic reactions are rare, but can induce an anaphylactic shock, even in patients who had tolerated the previous treatments.
- In very rare cases transfusion-related acute lung injury (TRALI) can occur after receiving immunoglobulins including Octagam 10%. This will lead to non-heart related accumulation of fluid in the air spaces of the lungs. You will recognize TRALI by severe difficulty in breathing, normal heart function and increased body temperature (fever). Symptoms typically appear within 1 to 6 hours after receiving treatment.
Tell your doctor or healthcare professional immediately if you notice such reactions during or after the infusion of Octagam 10%. He or she will decide whether to decrease the infusion rate or to stop the infusion completely or if further measures are necessary.
- Sometimes immunoglobulin solutions such as Octagam 10% can trigger a decrease in the number of white blood cells. Normally this condition resolves spontaneously within 1-2 weeks.
Virus safety
When medicines are made from human blood or plasma, certain measures are put in place to prevent infections being passed on to patients. These include:
- careful selection of blood and plasma donors to make sure those at risk of carrying infections are excluded
- testing of each donation and pools of plasma for signs of virus/infections
- steps included by the manufacturers in the processing of the blood or plasma that can inactivate or remove viruses.
Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of passing on infection cannot be totally excluded. This also applies to any unknown or emerging viruses or other types of infections.
The measures taken are considered effective for encapsulated viruses such as human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus.
The measures taken may be of limited value against non-encapsulated viruses such as hepatitis A virus and parvovirus B19.
Immunoglobulins have not been associated with hepatitis A or parvovirus B19 infections possibly because the antibodies against these infections, which are contained in the product, are protective.
Children and adolescents
There are no specific or additional warnings or precautions applicable for children and adolescents.
Other medicines and Octagam 10%
The infusion line may be flushed before and after administration of Octagam 10% with either normal saline or 5% dextrose in water.
Concomitant use of loop diuretics should be avoided.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription, or if you have received a vaccination in the last three months.
Octagam 10% may impair the effect of live attenuated virus vaccines such as measles, rubella, mumps and varicella.
After administration of this product, an interval of 3 months should elapse before vaccination with live attenuated virus vaccines. In the case of measles, this impairment may persist for up to 1 year.
Effects on blood tests
If you have a blood test after receiving Octagam 10%, please inform the person taking your blood or your doctor that you have received a human normal immunoglobulin solution, as this treatment may affect the results.
Blood Glucose Testing
Some types of blood glucose testing systems (so called glucometers) falsely interpret the maltose contained in Octagam 10% as glucose. This may result in falsely elevated glucose readings during an infusion and for a period of about 15 hours after the end of the infusion and, consequently, in the inappropriate administration of insulin, resulting in life-threatening hypoglycaemia (i.e. a decreased blood sugar level).
Also, cases of true hypoglycaemia may go untreated if the hypoglycaemic state is masked by falsely elevated glucose readings.
Accordingly, when administering Octagam 10% or other maltose-containing products, the measurement of blood glucose must be done with a test-system using a glucose-specific method. Systems based on the glucose dehydrogenase pyrroloquinolinequinone (GDH PQQ) or glucose-dye-oxidoreductase methods should not be used.
Review carefully the product information of the blood glucose testing system, including that of the test strips, to determine if the system is appropriate for use with maltose-containing parenteral products. If any uncertainty exists, please ask your treating physician to determine if the glucose testing system you are using is appropriate for use with maltose-containing parenteral products.
Octagam 10% with food, drink and alcohol
No effects have been observed. While using Octagam 10% adequate hydration before infusion should be taken into account.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking any medicine.
The safety of this medicinal product for use in human pregnancy has not been established in controlled clinical trials and therefore should only be given with caution to pregnant women and breast-feeding mothers. Immunoglobulin preparations have been shown to cross the placenta, increasingly during the third trimester. Clinical experience with immunoglobulins suggests that no harmful effects on the course of pregnancy, or on the foetus and the neonate are to be expected.
Immunoglobulins are excreted into human milk. No negative effects on the breastfed newborns/infants are anticipated.
Clinical experience with immunoglobulins suggests that no harmful effects on fertility are to be expected.
Driving and using machines
Octagam 10% has no or negligible influence on the ability to drive and use machines. However, patients who experience adverse reactions during treatment should wait for these to resolve before driving or operating machines.
Octagam 10% contains sodium
100 mL of this medicinal product contains 69 mg sodium (main component of cooking/table salt). This is equivalent to 3.45% of the recommended maximum daily intake of 2 g sodium for an adult.
To be taken into consideration by patients on a controlled sodium diet.
3 How to use Octagam 10%
Your doctor will decide if you need Octagam 10% and at what dose. Octagam 10% is administered as an intravenous infusion (infusion into a vein) by healthcare personnel. The dose and dosage regimen is dependent on the indication and may need to be individualised for each patient.
- If you have any further questions on the use of this product, ask your doctor or pharmacist.
Use in children and adolescents
The administration (intravenously) of Octagam 10% in children and adolescents (0-18 years) does not differ from the administration in adults.
4 Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Contact your doctor as soon as possible if you suffer from any of the serious side effects listed below (all are very rare and may affect up to 1 in 10,000 infusions).
In some cases, your doctor may need to interrupt treatment and reduce your dose or stop treatment:
- Swelling of the face, tongue and windpipe that can cause great difficulty in breathing
- A sudden allergic reaction with shortness of breath, rash, wheezing and drop of blood pressure
- Stroke that may cause weakness and / or loss of sensation down one side of the body
- Heart attack causing chest pain
- Blood clot causing pain and swelling of limbs
- Blood clot in lung causing chest pain and breathlessness
- Anaemia causing shortness of breath or looking pale
- Severe kidney disorder that may cause you to not pass urine
- A lung condition referred to as transfusion-related acute lung injury (TRALI) causing difficulty in breathing, bluish skin, fever, a decrease in blood pressure
If you experience any of the symptoms above, contact your doctor as soon as possible.
The following other very rare side effects have also been reported:
- Lack of white blood cells
- Fluid overload
- Too low sodium in blood
- Feeling agitated, anxious, confused or nervous
- Migraine
- Speech disorder
- Loss of consciousness
- Dizziness
- Tingling sensation in skin
- Reduced sense of touch or sensation
- Sensitivity to light
- Involuntary muscle contractions
- Impaired vision
- Angina pectoris
- Palpitations
- Changes in heart beat
- Temporary bluish lips or other parts of skin
- Circulatory collapse or shock
- Changes in blood pressure
- Vein inflammation
- Pale color of the skin
- Cough
- Breathing disorders
- Pulmonary oedema (accumulation of fluid in the lung)
- Bronchospasm (difficulty in breathing or wheezing)
- Respiratory failure
- Lack of oxygen in the blood
- Vomiting, diarrhoea, abdominal pain
- Hives, skin itching
- Redness of skin
- Skin rash
- Peeling of the skin
- Inflammation of the skin
- Hair loss
- Pains in joints or muscles
- Muscle weakness or stiffness
- Strong painful muscle contraction
- Neck pain, pain in legs or arms
- Kidney pain
- Swelling of the skin (oedema)
- Flushing, increased sweating
- Chest discomfort
- Flu-like symptoms
- Feeling cold or hot or generally unwell and weak
- Drowsiness
- Burning sensation
- Abnormalities in blood test reports of liver function
- False readings for blood sugar measurements
Common side effects (may affect up to 1 in 10 infusions):
- Hypersensitivity (allergic reaction)
- Headache
- Nausea
- Fever
- Feeling tired
- Skin reactions at injection site
Uncommon side effects (may affect up to 1 in 100 infusions):
- Eczema
- Back pain
- Chest pain
- Chills
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system (for details see below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
or search for MHRA Yellow Card in the Google Play or Apple App Store
5 How to store Octagam 10%
Keep this medicine out of the sight and reach of children.
Do not use Octagam 10% after the expiry date which is stated on the label and the carton.
Store in a refrigerator (2°C – 8°C). Keep the container in the outer carton in order to protect from light. Do not freeze.
The product may be removed from the refrigerator for a single period of up to 9 months (without exceeding the expiry date) and stored at a temperature below 25°C. At the end of this period, the product should not be refrigerated again and should be disposed of. The date at which the product was taken out of the refrigerator should be recorded on the outer carton.
Do not use Octagam 10% if you notice that the solution is cloudy, has deposits or is coloured intensively.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6 Contents of the pack and other information
What Octagam 10% contains
- The active substance is human normal immunoglobulin (human antibodies) 100 mg/ml (at least 95% is immunoglobulin G).
- The other ingredients are maltose and water for injections.
What Octagam 10% looks like and contents of the pack
Octagam 10% is a solution for infusion and is available in vials (2g/20ml) or bottles (5g/50ml, 6g/60ml, 10g/100ml, 20g/200ml).
Pack sizes:
2 g in 20 ml
5 g in 50 ml
6 g in 60 ml
10 g in 100 ml
20 g in 200 ml
3 x 10 g in 3 x 100 ml
3 x 20 g in 3 x 200 ml
The solution is clear or slightly opalescent, colourless or slightly yellow.
Not all pack sizes may be marketed.
Marketing authorisation holder
The Zenith Building
26 Spring Gardens
Manchester
M2 1AB
United Kingdom
Manufacturers
Oberlaaer Strasse 235
A-1100 Vienna
Austria
70-72 rue de Marèchal Foch
BP 33
F-67380 Lingolsheim
France
SE-112 75 Stockholm
Sweden
This medicinal product is authorised in the member states of the EEA under the following names:
Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Iceland, Latvia, Lithuania, Luxembourg, Malta, The Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Sweden, United Kingdom: Octagam
Italy: Gamten
Spain Octagamocta
This leaflet was last approved in 05/2019.
20190511
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: