- Anti-hestaminic & Respiratory Drugs (20)
- Anti-inflammatory Drugs (197) +-
- Baby & Mom (1346) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (52)
- Beauty (3129) +-
- Beauty > Skin Care > whitening (309)
- Chemotherapy & Immune Response (885) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (11)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (290)
- CIRCULATORY DISTURBANCE AGENTS (24)
- Diet & Fitness Products (284) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (191)
- HEMATOLOGY (39)
-
Medical Supplies (506)
+-
- Chemicals & Disinfectants (19)
- Dental Supplies (31)
- Devices & Instruments (11)
- Diabetic Supplies (121)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- KIDNEY UNIT SUPPLIES (21)
- Lab Supplies (3)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (2)
- Sanitary (5)
- Sterilization Supplies (1)
- Surgical Sutures (4)
- Syringes (3)
-
Medicines & Health (2727)
+-
- Allergy & Sinus (97)
- Children's Health Care (54)
- Cough, Cold & Flu (283)
- Digestive Health & Nausea (231)
- Ear, Nose & Throat Care (181)
- Eye Care (124)
- Feminine Care (323)
- Foot Care (12)
- Orthopaedic Appliances (1)
- Pain Relief & Management (244)
- Pill Organizer (2)
- Skin Treatments (863)
- Sleep & Snoring Aids (2)
- Support & Braces (8)
- Medicines & health > Gout releif (42)
- Natural & Organic Products (81) +-
- OTC > Analgesics > Anti-inflammatory Drugs (44)
-
Personal Care (3363)
+-
- Bath & Body (273)
- Deodorant & Anti-perspirants (191)
- Ear, Nose & Throat Care (177)
- Eye Care (131)
- Feminine Care (372)
- Foot Care (20)
- Hair Care (511)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (26)
- Massage & Relaxation (17)
- Natural & Organic Personal Care (7)
- Oral Care (91)
- Pregnancy & Fertility (64)
- Shaving & Grooming (75)
- Sun Care (80)
-
Prescription Drugs (2935)
+-
- Analgesics (184)
- Cardiovascular System (377)
- Drugs Affecting Musculoskeletal System (65)
- Drugs Used In Infections (56)
- Ear & Nose Drugs (2)
- Endocrine System (177)
- Gastrointestinal Tract (243)
- Gastrointestinal Tract > Hepatology > Liver treatment (60)
- GYNECOLOGY (2)
- Miscellaneous (11)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (47)
- NEUROLOGY (228)
- Nutrients & Blood Electrolytes (2)
- Respiratory System (154)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (115)
- Vaccines (1)
- Prescription drugs > Cardiovascular system > Anti-hypertension drugs (242)
- Sexual Wellness (304) +-
- Vitamins & Minerals Supplements (1230) +-
Ex Tax: 123EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

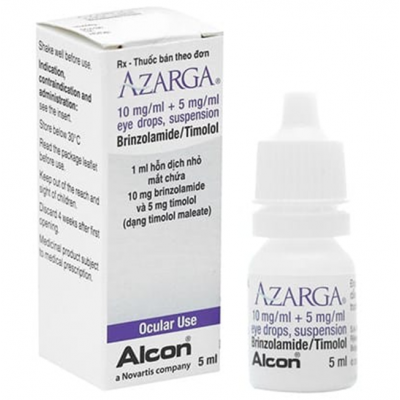
AZARGA ( brinzolamide 10 mg / ml + timolol 5 mg / ml ) eye drops 5 ml
Package leaflet: Information for the user
AZARGA 10 mg/ml + 5 mg/ml eye drops, suspension
brinzolamide/timolol
Read all of this leaflet carefully before you start using this medicine because it contains
important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them
even if their signs of illnesses are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side
effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What AZARGA is and what it is used for
2. What you need to know before you use AZARGA
3. How to use AZARGA
4. Possible side effects
5. How to store AZARGA
6. Contents of the pack and other information
1. What AZARGA is and what it is used for
AZARGA contains two active substances, brinzolamide and timolol, which work together to reduce
pressure within the eye.
AZARGA is used to treat high pressure in the eyes, also called glaucoma or ocular hypertension, in
adult patients that are more than 18 years of age and in whom high pressure in the eyes cannot be
controlled effectively by one medicine alone.
2. What you need to know before you use AZARGA
Do not use AZARGA
• If you are allergic to brinzolamide, medicines called sulphonamides (examples include
medicines used to treat diabetes, infections and also diuretics (water tablets)), timolol, betablockers (medicines used to lower blood pressure or to treat heart disease) or any of the other
ingredients of this medicine (listed in section 6).
• If you have now or have had in the past respiratory problems such as asthma, severe long lasting
obstructive bronchitis (severe lung condition which may cause wheezing, difficulty in breathing
and/or long standing cough) or other types of breathing problems.
• If you have severe hay fever
• If you have a slow heart beat, heart failure or disorders of heart rhythm (irregular heartbeats).
• If you have too much acidity in your blood (a condition called hyperchloraemic acidosis).
• If you have severe kidney problems.
Warnings and precautions
Only use AZARGA for dropping in your eye(s).
If signs of serious reactions or hypersensitivity occur, discontinue the use of this product and talk to
your doctor.
2
Talk to your doctor or pharmacist before using AZARGA if you have or have had in the past:
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness or
choking), heart failure, low blood pressure
• disturbances of heart rate such as slow heart beat
• breathing problems, asthma or chronic obstructive pulmonary disease
• poor blood circulation disease (such as Raynaud’s disease or Raynaud’s syndrome)
• diabetes as timolol may mask signs and symptoms of low blood sugar
• overactivity of the thyroid gland as timolol may mask signs and symptoms of thyroid disease
• muscular weakness (myasthenia gravis)
• tell your doctor before you have an operation that you are using AZARGA as timolol may
change effects of some medicines used during anaesthesia.
• if you have a history of atopy (a tendency to develop an allergic reaction) and severe allergic
reactions you may be more sensitive to developing an allergic reaction whilst using AZARGA
and adrenaline may not be as effective to treat an allergic reaction. When receiving any other
treatment please tell the doctor or nurse that you are taking AZARGA.
• if you have liver problems.
• if you have dry eyes or cornea problems.
• if you have problems with your kidneys.
Children and adolescents
AZARGA is not recommended for children and adolescents under 18 years.
Other medicines and AZARGA
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
AZARGA can affect, or be affected by, other medicines you are taking, including other eye drops for
the treatment of glaucoma. Tell your doctor if you are taking or intent to take medicines to lower blood
pressure like parasympathomimetics and guanetidine, or other heart medicines including quinidine
(used to treat heart conditions and some types of malaria), amiodarone or other medicines to treat heart
rhythm disorders and glycosides to treat heart insufficiency. Also tell your doctor if you are taking or
intend to take medicines to treat diabetes, or to treat gastric ulcers, antifungal, antiviral or antibiotic
medicines, or antidepressants such as fluoxetine and paroxetine.
If you are taking another carbonic anhydrase inhibitor (acetazolamide or dorzolamide), talk to your
doctor.
Increase in pupil size when taking Azarga and adrenaline (epinephrine) together has been reported
occasionally.
Pregnancy and breast-feeding
You should not use AZARGA if you are pregnant or might get pregnant, unless your doctor considers
it necessary. Talk to your doctor before you use AZARGA.
Do not use AZARGA if you are breast feeding, timolol may get into your milk.
Ask your doctor for advice before taking any medicine during breastfeeding.
Driving and using machines
Do not drive or use machines until your vision is clear. You may find that your vision is blurred for
some time just after using AZARGA.
One of the active ingredients may impair the ability to perform tasks requiring mental alertness and/or
physical coordination. If affected take care when driving or using machines.
3
AZARGA contains benzalkonium chloride
This medicine contains 3.34 µg benzalkonium chloride per drop (= 1 dose) which is equivalent to
0.01% or 0.1 mg/ml.
AZARGA contains a preservative (benzalkonium chloride) which may be absorbed by soft contact
lenses and may change the colour of the contact lenses. You should remove contact lenses before using
this medicine and put them back 15 minutes afterwards. Benzalkonium chloride may also cause eye
irritation, especially if you have dry eyes or disorders of the cornea (the clear layer at the front of the
eye). If you feel abnormal eye sensation, stinging or pain in the eye after using this medicine, talk to
your doctor.
3. How to use AZARGA
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or
pharmacist if you are not sure.
If you are changing from another eye drop medicine used to treat glaucoma to AZARGA, you should
stop using the other medicine and start using AZARGA the following day. Check with your doctor or
pharmacist if you are not sure
To prevent contamination of the dropper tip and the suspension, care must be taken not to touch
the eyelids, surrounding areas or other surfaces with the dropper tip. Keep the bottle tightly closed
when not in use.
The following measure is useful to limit the amount of medicine that will come into the blood after
application of eye drops:
- Keep the eyelid closed, while simultaneously applying gentle pressure to the corner of the eye
next to the nose with a finger for at least 2 minutes.
The recommended dose is
One drop in the affected eye or eyes, twice a day.
Only use AZARGA in both eyes if your doctor told you to. Take it for as long as your doctor told you
to.
How to use
• Get the AZARGA bottle and a mirror.
• Wash your hands.
• Shake well before use.
• Twist off the bottle cap. After the cap is removed, if the tamper evident snap collar is loose,
remove before using product.
• Hold the bottle, pointing down, between your thumb and fingers.
• Tilt your head back. Pull down your eyelid with a clean finger, until there is a ‘pocket’ between
the eyelid and your eye. The drop will go in here.
• Bring the bottle tip close to the eye. Use the mirror if it helps.
4
• Do not touch your eye or eyelid, surrounding areas or other surfaces with the dropper. It could
infect the drops.
• Gently press on the base of the bottle to release one drop of AZARGA at a time.
• Do not squeeze the bottle: it is designed so that a gentle press on the bottom is all that it needs.
• After using AZARGA, press a finger into the corner of your eye, by the nose for 2 minutes. This helps to stop AZARGA getting into the rest of the body.
• If you use drops in both eyes, repeat the steps for your other eye.
• Close the bottle cap firmly immediately after use.
• Use up one bottle before opening the next bottle.
If a drop misses your eye, try again.
If you are using other eye drop or eye ointment medicines leave at least 5 minutes between each
medicine. Eye ointments should be administered last.
If you use more AZARGA than you should, rinse your eye with warm water. Do not put in any more
drops until it is time for your next regular dose.
You may experience a decreased heart rate, decreased blood pressure, heart failure, difficulty
breathing and your nervous system may be affected
If you forget to use AZARGA, continue with the next dose as planned. Do not use a double dose to
make up for the forgotten dose. Do not use more than one drop in the affected eye(s) twice daily.
If you stop using AZARGA without speaking to your doctor, the pressure in your eye will not be
controlled which could lead to loss of sight.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects although not everybody gets them.
Stop using this medicine and contact your doctor immediately if you develop skin rash, severe skin
reaction, or severe redness and itching of the eye. These could be the signs of an allergic reaction
(frequency is not known).
You can usually carry on taking the drops, unless the effects are serious. If you are worried, talk to
your doctor or pharmacist. Do not stop using Azarga without speaking to your doctor first.
Common side effects (may affect up to 1 in 10 people)
• Effects in the eye: eye surface inflammation, blurred vision, signs and symptoms of eye
irritation (e.g. burning, stinging, itching, tearing, redness), eye pain.
• General side effects: heart rate decreased, taste disturbances.
Uncommon side effects (may affect up to 1 in 100 people)
• Effects in the eye:corneal erosion (damage to the front layer of the eyeball), Eye surface
inflammation with surface damage, inflammation inside the eye, corneal staining, abnormal
sensation in the eyes, eye discharge, dry eye, tired eyes, itchy eye, eye redness, eyelid redness.
• General side effects: decrease in white blood cell count, decreased blood pressure, cough,
blood in urine, body weakness.
5
Rare side effects (may affect up to 1 in 1,000 people)
• Effects in the eye: corneal disorder, sensitivity to light, increased tear production, eyelid
crusting
• General side effects: difficulty sleeping (insomnia), throat pain, running nose
Not known (frequency cannot be estimated from the available data)
• Effects in the eye: eye allergy, disturbance of vision, damage to the optic nerve, increased
pressure in eye, deposits on the eye surface, decreased eye sensation, inflammation or infection
of the conjunctiva (white of the eye), abnormal, double or reduced vision, increased
pigmentation of the eye, growth on surface of eye, eye swelling, sensitivity to light, decreased
growth or number of eyelashes, drooping of the upper eyelids (making the eye stay half closed),
inflammation of the eyelid and eye lid glands, inflammation in the cornea and detachment of the
layer below the retina that contains blood vessels following filtration surgery which may cause
visual disturbances, decreased corneal sensitivity.
• Heart and circulation: changes in rhythm or rate of the heartbeat, slow heart rate, palpitations,
a type of heart rhythm disorder, abnormal increase in heart rate, chest pain, reduced heart
function, heart attack, increased blood pressure, reduced blood supply to the brain, stroke,
oedema (fluid build up), congestive heart failure (heart disease with shortness of breath and
swelling of the feet and legs due to fluid build up), swelling of the extremities, low blood
pressure, discoloration of the fingers, toes, and occasionally other areas of the body (Raynaud’s
phenomenon), cold hands and feet.
• Respiratory: Constriction of the airways in the lungs (predominantly in patients with preexisting disease) shortness of breath or difficulty breathing, cold symptoms, chest congestion,
sinus infection, sneezing, stuffy nose, dry nose, nose bleeds, asthma, throat irritation.
• Nervous system and general disorders: hallucinations, depression, nightmares, memory loss,
headache, nervousness, irritability, tiredness, shaking, feeling abnormal, fainting, dizziness,
drowsiness, generalised or severe weakness, unusual sensations like pins and needles.
• Gastric: nausea, vomiting, diarrhoea, intestinal gas or abdominal discomfort, inflammation of
the throat, dry or abnormal sensation in mouth, indigestion, stomach ache.
• Blood: abnormal liver function values, increased blood chlorine levels, or decreased red blood
cell count as seen in a blood test.
• Allergy: increased allergic symptoms, generalised allergic reactions including swelling beneath
the skin that can occur in areas such as the face and limbs and can obstruct the airway which
may cause difficulty swallowing or breathing, hives, localised and generalised rash, itchiness,
severe sudden life-threatening allergic reaction.
• Ear: ringing in the ears, sensation of spinning or dizziness.
• Skin: rash, skin redness or inflammation, abnormal or decreased skin sensation, hair loss, rash
with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis.
• Muscular: generalised back, joint, or muscle pain not caused by exercise, muscle spasms, pain
in extremities, muscle weakness/tiredness, increases in the signs and symptoms of myasthenia
gravis (muscle disorder).
• Kidney: kidney pain such as lower back pain, frequent urination.
• Reproduction: sexual dysfunction, decreased libido, male sexual difficulty.
• Metabolism: low blood sugar levels.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects
not listed in this leaflet. You can also report side effects directly (see details below). By reporting side
effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme
6
Website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or
Apple App Store
5. How to store AZARGA
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle and the carton after EXP.
The expiry date refers to the last day of that month.
This medicine does not require any special storage conditions.
Throw away the bottle 4 weeks after first opening to prevent infections, and use a new bottle. Write
down the date of opening on the bottle label and carton label in the space provided.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to
throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What AZARGA contains
• The active substances are brinzolamide and timolol. One ml of suspension contains 10 mg of
brinzolamide and 5 mg of timolol (as maleate).
• The other ingredients are benzalkonium chloride (see section 2 ‘AZARGA contains
benzalkonium’), carbopol 974P, disodium edetate, mannitol (E421), purified water, sodium
chloride, tyloxapol, hydrochloric acid and/or sodium hydroxide.
Tiny amounts of hydrochloric acid and/or sodium hydroxide are added to keep acidity levels
(pH levels) normal.
What AZARGA looks like and contents of the pack
AZARGA is a liquid (white to off-white uniform suspension) supplied in a pack containing one 5 ml
plastic bottle with a screw cap or in a pack containing three 5 ml bottles.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
S.A. Alcon - Couvreur N.V.
Rijksweg 14
B-2870 Puurs
Belgium
For any information about this medicine, please contact the local representative of the Marketing
Authorisation Holder.
United Kingdom
Novartis Pharmaceuticals UK Ltd.
7
Tel: +44 1276 698370
This leaflet was last revised in 07/2020
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site:
http://www.ema.europa.eu
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below:










![OCUGUARD LX ANTIOXIDANT FOR EYE HEALTH ( VIT. C 60MG + VIT. E 13.5MG + ZINC 15MG + COPPER 2MG + MARIGOLD FLOWER EXTRACT [ NLT 20% LUTEIN + NLT 4% ZEAXANTHIN ] 6MG ) 30 CAPSULES](http://egyptiandrugstore.com/image/cache/data/MANAR 21/OCUGAURD LX-300x300.png)