- Anti-hestaminic & Respiratory Drugs (20)
- Anti-inflammatory Drugs (192) +-
- Baby & Mom (1279) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (53)
- Beauty (2738) +-
- Beauty > Skin Care > whitening (273)
- Chemotherapy & Immune Response (855) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (11)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (283)
- CIRCULATORY DISTURBANCE AGENTS (23)
- Diet & Fitness Products (272) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (188)
- HEMATOLOGY (39)
-
Medical Supplies (496)
+-
- Chemicals & Disinfectants (19)
- Dental Supplies (26)
- Devices & Instruments (10)
- Diabetic Supplies (119)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- KIDNEY UNIT SUPPLIES (21)
- Lab Supplies (3)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (2)
- Sanitary (5)
- Sterilization Supplies (0)
- Surgical Sutures (4)
- Syringes (3)
-
Medicines & Health (2523)
+-
- Allergy & Sinus (94)
- Children's Health Care (51)
- Cough, Cold & Flu (270)
- Digestive Health & Nausea (220)
- Ear, Nose & Throat Care (177)
- Eye Care (116)
- Feminine Care (319)
- Foot Care (4)
- Orthopaedic Appliances (0)
- Pain Relief & Management (230)
- Pill Organizer (2)
- Skin Treatments (739)
- Sleep & Snoring Aids (0)
- Support & Braces (6)
- Medicines & health > Gout releif (42)
- Natural & Organic Products (82) +-
- OTC > Analgesics > Anti-inflammatory Drugs (45)
-
Personal Care (3077)
+-
- Bath & Body (256)
- Deodorant & Anti-perspirants (179)
- Ear, Nose & Throat Care (173)
- Eye Care (122)
- Feminine Care (366)
- Foot Care (12)
- Hair Care (408)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (21)
- Massage & Relaxation (18)
- Natural & Organic Personal Care (7)
- Oral Care (82)
- Pregnancy & Fertility (63)
- Shaving & Grooming (65)
- Sun Care (68)
-
Prescription Drugs (2804)
+-
- Analgesics (182)
- Cardiovascular System (366)
- Drugs Affecting Musculoskeletal System (63)
- Drugs Used In Infections (54)
- Ear & Nose Drugs (2)
- Endocrine System (173)
- Gastrointestinal Tract (235)
- Gastrointestinal Tract > Hepatology > Liver treatment (59)
- GYNECOLOGY (2)
- Miscellaneous (11)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (46)
- NEUROLOGY (216)
- Nutrients & Blood Electrolytes (2)
- Respiratory System (150)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (41)
- Vaccines (1)
- Prescription drugs > Cardiovascular system > Anti-hypertension drugs (236)
- Sexual Wellness (293) +-
- Vitamins & Minerals Supplements (1145) +-
Ex Tax: 444EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

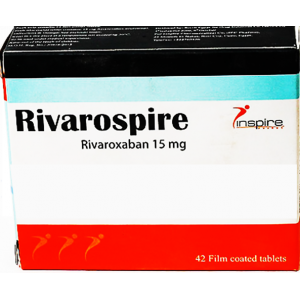
Dosage Forms & Strengths
tablet
2.5mg
5mg
Stroke Prophylaxis with Atrial Fibrillation
To prevent stroke and systemic embolism in nonvalvular atrial fibrillation
5 mg PO BID
Renal impairment (nonvalvular atrial fibrillation)
- Mild-to-moderate: No dosage adjustment required
- Serum creatinine ≥1.5 mg/dL: Decrease dose to 2.5 mg BID if patient has 1 additional characteristic of age ≥80 years or weight ≤60 kg
- ESRD maintained on hemodialysis: 5 mg BID; decrease dose to 2.5 mg BID if 1 additional characteristic of age ≥80 years or weight ≤60 kg is present
Postoperative Prophylaxis of DVT/PE
Indicated following hip or knee replacement surgery
Initial: Give 2.5 mg PO 12-24 hr after surgery
Duration of therapy (hip replacement): 2.5 mg PO BID for 35 days
Duration of therapy (knee replacement): 2.5 mg PO BID for 12 days
Renal impairment, including with ESRD on dialysis
- Deep Vein Thrombosis: No dose adjustment recommended; not studied in ESRD on dialysis or patients with a CrCl <15 mL/min; dosing recommendations based on pharmacokinetic and pharmacodynamic (anti-FXa activity) data in study subjects with ESRD maintained on dialysis
DVT or PE Treatment
- Indicated for treatment of deep venous thrombosis (DVT) and pulmonary embolism (PE)
10 mg PO BID x 7 days, then 5 mg BID
*Reduce risk for recurrent DVT or PE:
Indicated to reduce the risk of recurrent DVT and PE following initial 6 months treatment for DVT and/or PE
2.5 mg PO BID
*Renal impairment, including with ESRD:
- Deep Vein Thrombosis: No dose adjustment recommended; not studied in ESRD on dialysis or patients with a CrCl <15 mL/min; dosing recommendations based on pharmacokinetic and pharmacodynamic (anti-FXa activity) data in study subjects with ESRD maintained on dialysis
Dosage Modifications
Coadministration with dual inhibitors of CYP3A4 and P-gp
- If taking >2.5 PO BID, decrease dose by 50%
- If taking 2.5 mg BID, avoid coadministration with strong dual inhibitors
Nonvalvular atrial fibrillation
- Decrease dose to 2.5 mg PO BID in patients with any 2 of the following characteristics:
- Age ≥80 years
- Weight ≤60 kg
- Serum creatinine ≥1.5 mg/dL
Hepatic impairment
- Mild: No dosage adjustment required
- Moderate: Patients may have intrinsic coagulation abnormalities; data are limited and no recommendations are available
- Severe: Not recommended
Dosing Considerations
Switching between apixaban and anticoagulants other than warfarin: Discontinue one being taken, and begin the other at the next scheduled dose
Switching from warfarin to apixaban: Discontinue warfarin and initiate apixaban when INR <2.0
Switching from apixaban to warfarin
- Apixaban affects INR, so measurements during coadministration with warfarin may not determine appropriate warfarin dose
- If continuous anticoagulation is necessary, discontinue apixaban and begin both a parenteral anticoagulant and warfarin at the time the next dose of apixaban would have been taken
- Discontinue parenteral anticoagulant when INR reaches an acceptable level
Surgery/procedures
- Discontinue at least 48 hr before elective surgery or invasive procedures with a moderate or high risk of unacceptable or clinically significant bleeding
- Discontinue at least 24 hr before elective surgery or invasive procedures with low risk of unacceptable bleeding or where bleeding would be noncritical in location and easily controlled
Interactions
Contraindicated (13)
betrixaban
carbamazepine
defibrotide
dexamethasone
fondaparinux
fosphenytoin
mifepristone
omacetaxine
phenytoin
prothrombin complex concentrate, human
rifabutin
St John's Wort
vorapaxar
Serious (72)
abciximab
alteplase
alteplase and apixaban both increase anticoagulation. Avoid or Use Alternate Drug.
anagrelide
antithrombin alfa
apalutamide
argatroban
atazanavir
bivalirudin
caplacizumab
celecoxib
cilostazol
clopidogrel
conivaptan
dabigatran
dalteparin
darunavir
desirudin
desvenlafaxine
diclofenac
dipyridamole
duloxetine
edoxaban
enoxaparin
enzalutamide
eptifibatide
etodolac
Factor X, human
fenoprofen
fexinidazole
flurbiprofen
heparin
ibuprofen
ibuprofen IV
idelalisib
indomethacin
ivosidenib
ketoconazole
ketoprofen
ketorolac
lasmiditan
levoketoconazole
levomilnacipran
lonafarnib
meclofenamate
mefenamic acid
meloxicam
milnacipran
nabumetone
naproxen
nelfinavir
nirmatrelvir/ritonavir
oxaprozin
phenobarbital
piroxicam
posaconazole
prasugrel
primidone
protein C concentrate
quinidine
reteplase
rifampin
ritonavir
rivaroxaban
sotorasib
sulindac
tenecteplase
tepotinib
ticlopidine
tirofiban
tolmetin
venlafaxine
warfarin
Monitor Closely (56)
acalabrutinib
aspirin
belzutifan
berotralstat
cenobamate
ceritinib
citalopram
cobicistat
crofelemer
dabrafenib
danicopan
diltiazem
dronedarone
efavirenz
elagolix
eliglustat
enasidenib
encorafenib
escitalopram
fedratinib
fish oil triglycerides
fluoxetine
fluvoxamine
glecaprevir/pibrentasvir
ibrutinib
icosapent
iloperidone
imatinib
istradefylline
itraconazole
larotrectinib
lenacapavir
lonafarnib
lopinavir
lorlatinib
mavorixafor
melatonin
mifepristone
mitotane
nefazodone
nintedanib
paroxetine
ribociclib
rucaparib
sarecycline
saw palmetto
sertraline
stiripentol
tazemetostat
tecovirimat
tipranavir
tucatinib
verapamil
voriconazole
vortioxetine
zanubrutinib
Minor (6)
acetazolamide
anastrozole
clarithromycin
cyclophosphamide
danazol
drospirenone
Administration
Images
Patient Handout
Formulary
Dosing & Uses
Adult
Pediatric
Dosage Forms & Strengths
tablet
2.5mg
5mg
Stroke Prophylaxis with Atrial Fibrillation
To prevent stroke and systemic embolism in nonvalvular atrial fibrillation
5 mg PO BID
Renal impairment (nonvalvular atrial fibrillation)
Mild-to-moderate: No dosage adjustment required
Serum creatinine ≥1.5 mg/dL: Decrease dose to 2.5 mg BID if patient has 1 additional characteristic of age ≥80 years or weight ≤60 kg
ESRD maintained on hemodialysis: 5 mg BID; decrease dose to 2.5 mg BID if 1 additional characteristic of age ≥80 years or weight ≤60 kg is present
Postoperative Prophylaxis of DVT/PE
Indicated following hip or knee replacement surgery
Initial: Give 2.5 mg PO 12-24 hr after surgery
Duration of therapy (hip replacement): 2.5 mg PO BID for 35 days
Duration of therapy (knee replacement): 2.5 mg PO BID for 12 days
Renal impairment, including with ESRD on dialysis
Deep Vein Thrombosis: No dose adjustment recommended; not studied in ESRD on dialysis or patients with a CrCl <15 mL/min; dosing recommendations based on pharmacokinetic and pharmacodynamic (anti-FXa activity) data in study subjects with ESRD maintained on dialysis
DVT or PE Treatment
Indicated for treatment of deep venous thrombosis (DVT) and pulmonary embolism (PE)
10 mg PO BID x 7 days, then 5 mg BID
Reduce risk for recurrent DVT or PE
Indicated to reduce the risk of recurrent DVT and PE following initial 6 months treatment for DVT and/or PE
2.5 mg PO BID
Renal impairment, including with ESRD
Deep Vein Thrombosis: No dose adjustment recommended; not studied in ESRD on dialysis or patients with a CrCl <15 mL/min; dosing recommendations based on pharmacokinetic and pharmacodynamic (anti-FXa activity) data in study subjects with ESRD maintained on dialysis
Dosage Modifications
Coadministration with dual inhibitors of CYP3A4 and P-gp
If taking >2.5 PO BID, decrease dose by 50%
If taking 2.5 mg BID, avoid coadministration with strong dual inhibitors
Nonvalvular atrial fibrillation
Decrease dose to 2.5 mg PO BID in patients with any 2 of the following characteristics:
Age ≥80 years
Weight ≤60 kg
Serum creatinine ≥1.5 mg/dL
Hepatic impairment
Mild: No dosage adjustment required
Moderate: Patients may have intrinsic coagulation abnormalities; data are limited and no recommendations are available
Severe: Not recommended
Dosing Considerations
Switching between apixaban and anticoagulants other than warfarin: Discontinue one being taken, and begin the other at the next scheduled dose
Switching from warfarin to apixaban: Discontinue warfarin and initiate apixaban when INR <2.0
Switching from apixaban to warfarin
Apixaban affects INR, so measurements during coadministration with warfarin may not determine appropriate warfarin dose
If continuous anticoagulation is necessary, discontinue apixaban and begin both a parenteral anticoagulant and warfarin at the time the next dose of apixaban would have been taken
Discontinue parenteral anticoagulant when INR reaches an acceptable level
Surgery/procedures
Discontinue at least 48 hr before elective surgery or invasive procedures with a moderate or high risk of unacceptable or clinically significant bleeding
Discontinue at least 24 hr before elective surgery or invasive procedures with low risk of unacceptable bleeding or where bleeding would be noncritical in location and easily controlled
Interactions
Interaction Checker
Enter a drug name to check for any interactions.
Type a drug, OTC, or herbal name
+ apixaban
All InteractionsSort By:
Severity
Contraindicated (13)
betrixaban
carbamazepine
defibrotide
dexamethasone
fondaparinux
fosphenytoin
mifepristone
omacetaxine
phenytoin
prothrombin complex concentrate, human
rifabutin
St John's Wort
vorapaxar
Serious (72)
abciximab
alteplase
alteplase and apixaban both increase anticoagulation. Avoid or Use Alternate Drug.
anagrelide
antithrombin alfa
apalutamide
argatroban
atazanavir
bivalirudin
caplacizumab
celecoxib
cilostazol
clopidogrel
conivaptan
dabigatran
dalteparin
darunavir
desirudin
desvenlafaxine
diclofenac
dipyridamole
duloxetine
edoxaban
enoxaparin
enzalutamide
eptifibatide
etodolac
Factor X, human
fenoprofen
fexinidazole
flurbiprofen
heparin
ibuprofen
ibuprofen IV
idelalisib
indomethacin
ivosidenib
ketoconazole
ketoprofen
ketorolac
lasmiditan
levoketoconazole
levomilnacipran
lonafarnib
meclofenamate
mefenamic acid
meloxicam
milnacipran
nabumetone
naproxen
nelfinavir
nirmatrelvir/ritonavir
oxaprozin
phenobarbital
piroxicam
posaconazole
prasugrel
primidone
protein C concentrate
quinidine
reteplase
rifampin
ritonavir
rivaroxaban
sotorasib
sulindac
tenecteplase
tepotinib
ticlopidine
tirofiban
tolmetin
venlafaxine
warfarin
Monitor Closely (56)
acalabrutinib
aspirin
belzutifan
berotralstat
cenobamate
ceritinib
citalopram
cobicistat
crofelemer
dabrafenib
danicopan
diltiazem
dronedarone
efavirenz
elagolix
eliglustat
enasidenib
encorafenib
escitalopram
fedratinib
fish oil triglycerides
fluoxetine
fluvoxamine
glecaprevir/pibrentasvir
ibrutinib
icosapent
iloperidone
imatinib
istradefylline
itraconazole
larotrectinib
lenacapavir
lonafarnib
lopinavir
lorlatinib
mavorixafor
melatonin
mifepristone
mitotane
nefazodone
nintedanib
paroxetine
ribociclib
rucaparib
sarecycline
saw palmetto
sertraline
stiripentol
tazemetostat
tecovirimat
tipranavir
tucatinib
verapamil
voriconazole
vortioxetine
zanubrutinib
Minor (6)
acetazolamide
acetazolamide will increase the level or effect of apixaban by affecting hepatic/intestinal enzyme CYP3A4 metabolism. Minor/Significance Unknown.
anastrozole
clarithromycin
cyclophosphamide
danazol
drospirenone
Adverse Effects
Bleeding (Aristotle Study)
Major (2.13%, warfarin 3.09%; P <0.0001)
GI (0.83%, warfarin 0.93%)
Intracranial (0.33%, warfarin 0.82%)
Intraocular (0.06%, warfarin 0.14%)
Fatal (0.06%, warfarin 0.24%)
Clinically relevant nonmajor bleeding (2.08%, warfarin 3.0%; P <0.0001)
Bleeding (Averroes Study)
Major (1.41%, aspirin 0.92%; P = 0.07)
Fatal (0.16%, aspirin 0.16%)
Intracranial (0.34%, aspirin 0.35%)
<1%
Hypersensitivity reactions (including skin rash and anaphylactic reactions such as allergic edema)
Syncope
Postmarketing Reports
Blood and lymphatic system disorders: Thrombocytopenia (including platelet count decreases)
Vascular disorders: Hypotension (including procedural hypotension)
Respiratory, thoracic, and mediastinal disorders: Epistaxis
Gastrointestinal disorders: Gastrointestinal hemorrhage (including hematemesis and melena), hematochezia
Hepatobiliary disorders: Liver function test abnormal, blood alkaline phosphatase increased, blood bilirubin increased
Renal and urinary disorders: Hematuria (including respective laboratory parameters)
Injury, poisoning, and procedural complications: Wound secretion, incision-site hemorrhage (including incision-site hematoma), operative hemorrhage
Warnings
| Black Box Warnings Discontinuing in patients with nonvalvular atrial fibrillation Premature discontinuation of any oral anticoagulant, including, apixaban, increases risk of thrombotic events Consider using another anticoagulant if anticoagulation with apixaban is discontinued for a reason other than pathological bleeding or completion of a course of therapy An increased rate of stroke was observed following discontinuation of apixaban in clinical trials in patients with nonvalvular atrial fibrillation If anticoagulation with apixaban must be discontinued for a reason other than pathological bleeding, coverage with another anticoagulant should be strongly considered (see Dosing Considerations) Spinal/epidural hematoma Increased risk of epidural or spinal hematoma when used with neuraxial anesthesia (epidural/spinal anesthesia) or spinal puncture (can result in long-term or permanent paralysis) Risk increased with indwelling epidural catheters for administration of analgesia or by the concomitant use of drugs affecting hemostasis (eg, NSAIDs, platelet aggregation inhibitors, other anticoagulants) Risk also increased by traumatic or repeated epidural or spinal puncture; if this occurs, delay apixaban administration for 48 hr Monitor patients for signs and symptoms of neurologic impairment; if neurologic compromise is noted, urgent treatment is necessary Indwelling epidural or intrathecal catheters should not be removed earlier than 24 hr after the last administration of apixaban; the next apixaban dose should not be administered earlier than 5 hr after the removal of the catheter Consider the potential benefit versus risk before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis |
Contraindications
Severe hypersensitivity (ie, anaphylactic reactions)
Active pathological bleeding
Cautions
Discontinuing apixaban in the absence of adequate alternative anticoagulation increases the risk of thrombotic events (see Black Box Warnings)
Risk of epidural or spinal hematoma when used with neuraxial anesthesia (see Black Box Warnings)
Safety and efficacy has not been studied in patients with prosthetic heart valves; therefore, use of is not recommended in these patients
Not recommended as an alternative to unfractionated heparin for the initial treatment of PE in patients who present with hemodynamic instability or who may receive thrombolysis or pulmonary embolectomy
Coadministration with strong dual inhibitors of CYP3A4 and P-gp (see Dosage Modifications)
Avoid coadministration with strong dual inducers of CYP3A4 and P-gp; such drugs decrease apixaban’s systemic exposure
Increases the risk of bleeding and can cause serious, potentially fatal, bleeding; advise patients of signs and symptoms of blood loss and to report them immediately or go to an emergency room; discontinue therapy in patients with active pathological hemorrhage
Coadministration with other drugs that affect hemostasis increases bleeding risk (eg, aspirin and other antiplatelet agents, other anticoagulants, heparin, thrombolytic agents, SSRIs, SNRIs, NSAIDs)
Prolongs PT and aPTT; however, changes are small and highly variable and are not useful for monitoring anticoagulation effect of apixaban
Direct-acting oral anticoagulants (DOACs), are not recommended for use in patients with triple-positive antiphospholipid syndrome (APS); for patients with APS (especially those who are triple positive [positive for lupus anticoagulant, anticardiolipin, and anti–beta 2- glycoprotein I antibodies]), treatment with DOACs has been associated with increased rates of recurrent thrombotic events compared with vitamin K antagonist therapy
Reversing apixaban effect
- Anticoagulant effect is expected to persist for about 24 hr after the last dose (~2 half-lives)
- Coagulation factor Xa recombinant, inactivated-zhzo is commercially available for reversal of the anticoagulant effect of apixaban when reversal of anticoagulation is needed because of life-threatening or uncontrolled bleeding
- Because of high plasma protein binding, apixaban is not expected to be dialyzable
- Protamine sulfate and vitamin K would not be expected to affect the anticoagulant activity of apixaban
- There is no experience with antifibrinolytic agents (tranexamic acid, aminocaproic acid) in individuals receiving apixaban
- There is neither scientific rationale for reversal nor experience with systemic hemostatics (desmopressin and aprotinin) in individuals receiving apixaban
- Use of procoagulant reversal agents (eg, prothrombin complex concentrate, activated prothrombin complex concentrate, or recombinant factor VIIa) may be considered but has not been evaluated in clinical studies
- Activated oral charcoal reduces absorption of apixaban, thereby lowering plasma concentration
Pregnancy & Lactation
Pregnancy
There are no adequate and well-controlled studies in pregnant women
Treatment is likely to increase the risk of hemorrhage during pregnancy and delivery
Use of anticoagulants, during pregnancy, may increase risk of bleeding in fetus and neonate
Pregnancy confers an increased risk of thromboembolism that is higher for women with underlying thromboembolic disease and certain high-risk pregnancy conditions
Published data describe that women with a previous history of venous thrombosis are at high risk for recurrence during pregnancy
Therapy should be administered during pregnancy only if the potential benefit outweighs the potential risk to the mother and fetus
Animal studies
Treatment of pregnant rats, rabbits, and mice after implantation until the end of gestation resulted in fetal exposure to apixaban, but was not associated with increased risk for fetal malformations or toxicity
Labor and delivery
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding; use during labor or delivery in women who are receiving neuraxial anesthesia may result in epidural or spinal hematomas; consider use of a shorter acting anticoagulant as delivery approaches
Consider the risks of bleeding and of stroke in this setting
Females of reproductive potential
Females of reproductive potential requiring anticoagulation should discuss pregnancy planning with their physician
The risk of clinically significant uterine bleeding, potentially requiring gynecological surgical interventions, identified with oral anticoagulants should be assessed in females of reproductive potential and those with abnormal uterine bleeding
Lactation
There are no data on presence of drug metabolites in human milk, effects on breastfed child, or the effects on milk production; the drug and/or its metabolites were present in milk of rats
Rats excrete apixaban in milk (12% of the maternal dose)
Because human exposure through milk is unknown, instruct women to either discontinue breastfeeding or to discontinue apixaban therapy, taking into account the importance of the drug to the mother
Pregnancy Categories
A: Generally acceptable. Controlled studies in pregnant women show no evidence of fetal risk.
B: May be acceptable. Either animal studies show no risk but human studies not available or animal studies showed minor risks and human studies done and showed no risk.
C: Use with caution if benefits outweigh risks. Animal studies show risk and human studies not available or neither animal nor human studies done.
D: Use in LIFE-THREATENING emergencies when no safer drug available. Positive evidence of human fetal risk.
X: Do not use in pregnancy. Risks involved outweigh potential benefits. Safer alternatives exist.
NA: Information not available.
Pharmacology
Mechanism of Action
Factor Xa inhibitor that inhibits platelet activation by selectively and reversibly blocking the active site of factor Xa without requiring a cofactor (eg, antithrombin III) for activity
Inhibits free and clot-bound factor Xa, and prothrombinase activity; no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin
Blood coagulation cascade is dependent on the activation of factor X to factor Xa via the intrinsic and extrinsic pathways, which play a central role in the blood coagulation cascade
Absorption
Bioavailability: Displays prolonged absorption Peak Plasma Concentration: 3-4 hr
Distribution
Protein Bound: 87%
Vdss: 21 L
Metabolism
Metabolized mainly by CYP3A4
Metabolized with minor contributions from CYP1A2, 2C8, 2C9, 2C19 and 2J2
Major sites of biotransformation: O-demethylation and hydroxylation at the 3-oxopirperidinyl moiety
Metabolites: No active circulating metabolites Substrate of P-gp and BCRP
Elimination
Half-life: 5-6 hr (dominant); 12 hr (apparent half-life with repeated dosing)
Dialyzable: No
Renal clearance: 27%
Excretion: 25% in urine and feces as metabolites; renal excretion accounts for 27% of total clearance; biliary and direct intestinal excretion contributes to elimination in feces
Administration
Oral Administration
May take with or without food
Missed dose:
- If not taken at the scheduled time, the dose should be taken as soon as possible on the same day and twice daily administration should be resumed
- Do not double the dose to make up for a missed dose
- Unable to swallow whole tablets
- 5 mg and 2.5 mg tablets may be crushed and suspended in water, 5% dextrose in water (D5W), or apple juice, or mixed with applesauce and promptly administered orally
- Alternatively, tablets may be crushed and suspended in 60 mL of water or D5W and promptly delivered through a nasogastric tube
- Crushed tablets are stable in water, D5W, apple juice, and applesauce for up to 4 hr
Patient Education
apixaban oral
APIXABAN - ORAL
(a-PIX-a-ban)
COMMON BRAND NAME(S):
Eliquis
WARNING: Do not stop taking apixaban unless directed by your doctor. If you stop taking this medication early, you have a higher risk of forming a serious blood clot (such as a stroke, blood clot in the legs/lungs). Your doctor may direct you to take a different "blood thinning" or antiplatelet medication to reduce your risk. Get medical help right away if you have weakness on one side of the body, trouble speaking, sudden vision changes, confusion, chest pain, trouble breathing, or pain/warmth/swelling in the legs.People taking this medication may bleed near the spinal cord after certain spinal procedures. Bleeding in this area can cause paralysis that lasts a long time or could become permanent. Before any spinal procedure, ask your doctor about the benefits and risks. The risk of bleeding may be higher if you have a deformed spine, or have had spinal procedures/surgery before (such as epidural catheter placement, difficult epidural/spinal puncture), or are taking other drugs that can cause bleeding/bruising (including antiplatelet drugs such as clopidogrel, "blood thinners" such as warfarin/enoxaparin, nonsteroidal anti-inflammatory drugs-NSAIDs such as ibuprofen). Tell your doctor right away if you notice symptoms such as back pain, leg numbness/tingling/weakness, loss of control of the bowels or bladder (incontinence).
USES:
Apixaban is used to prevent serious blood clots from forming due to a certain irregular heartbeat (atrial fibrillation) or after hip/knee replacement surgery. With atrial fibrillation, part of the heart does not beat the way it should. This can lead to blood clots forming, which can travel to other parts of your body (such as the lungs or legs) or increase your risk for stroke. In the United States, apixaban is also approved to treat certain types of blood clots (deep vein thrombosis-DVT, pulmonary embolus-PE) and to prevent them from forming again.Apixaban is an anticoagulant that works by blocking certain clotting proteins in your blood.
HOW TO USE:
See also Warning section.Read the Medication Guide and, if available, the Patient Information Leaflet provided by your pharmacist before you start taking apixaban and each time you get a refill. If you have any questions, ask your doctor or pharmacist.Take this medication by mouth with or without food as directed by your doctor, usually twice daily (every 12 hours). If you cannot swallow the tablet whole, you may crush the tablet and mix with water, apple juice, or applesauce and take it right away.The dosage is based on your medical condition, age, weight, response to treatment, and other medications you may be taking. Be sure to tell your doctor and pharmacist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products). If you are taking apixaban to prevent blood clots from forming after surgery, the length of treatment is based on the type of surgery that you had.Do not stop taking this medication without consulting your doctor. Some conditions may become worse when this drug is suddenly stopped. Do not run out of this medication. Order your refills early to avoid running out of pills.Use this medication regularly to get the most benefit from it. To help you remember, take it at the same times each day.
SIDE EFFECTS:
See also Warning section.Nausea, easy bruising, or minor bleeding (such as nosebleed, bleeding from cuts) may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.Remember that this medication has been prescribed because your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.This medication can cause serious bleeding if it affects your blood clotting proteins too much. Tell your doctor right away if you have any signs of serious bleeding, including: nosebleeds that happen often or don't stop, unusual tiredness/weakness, unusual pain/swelling/discomfort, unusual bruising, prolonged bleeding from cuts or gums, unusually heavy/prolonged menstrual flow, pink/dark urine, coughing up blood, vomit that is bloody or looks like coffee grounds, severe headache, dizziness/fainting, bloody/black/tarry stools, difficulty swallowing.Get medical help right away if you have any signs of very serious bleeding, including: vision changes, confusion, trouble speaking, weakness on one side of the body.A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.In the US -Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
PRECAUTIONS:
Before taking apixaban, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.Before using this medication, tell your doctor or pharmacist your medical history, especially of: liver disease, kidney disease, bleeding problems (such as bleeding of the stomach/intestines, bleeding in the brain), blood disorders (such as anemia, hemophilia, thrombocytopenia), recent major injury/surgery, stroke, a certain clotting disorder (antiphospholipid syndrome), frequent falls/injuries.Before having surgery or any medical/dental procedures (especially spinal puncture or spinal/epidural anesthesia), tell your doctor or dentist that you are taking this medication and about all the products you use (including prescription drugs, nonprescription drugs, and herbal products). Your doctor or dentist may tell you to stop taking apixaban before your surgery. Ask for specific instructions about stopping or starting this medication.This medication may cause stomach bleeding. Daily use of alcohol while using this medicine will increase your risk for stomach bleeding. Limit alcoholic beverages. Ask your doctor or pharmacist about how much alcohol you may safely drink.This medication can cause heavy bleeding. To lower the chance of getting cut, bruised, or injured, use caution with sharp objects like razors and nail cutters, and avoid activities such as contact sports. Use an electric razor when shaving and a soft toothbrush when brushing your teeth. If you fall or injure yourself, especially if you hit your head, call your doctor right away. Your doctor may need to check you.During pregnancy, this medication should be used only when clearly needed. Discuss the risks and benefits with your doctor.It is unknown if this medication passes into breast milk. Because of the possible risk to the infant, breastfeeding is not recommended while using this medication. Consult your doctor before breastfeeding.
DRUG INTERACTIONS:
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.Some products that may interact with this drug include: mifepristone, other drugs that can cause bleeding/bruising (including antiplatelet drugs such as clopidogrel, NSAIDs such as ibuprofen/naproxen, "blood thinners" such as warfarin/enoxaparin), certain antidepressants (including SSRIs such as fluoxetine, SNRIs such as desvenlafaxine/venlafaxine).Other medications can affect the removal of apixaban from your body, which may affect how apixaban works. Examples include certain azole antifungals (such as itraconazole, ketoconazole, posaconazole), conivaptan, HIV protease inhibitors (such as lopinavir), rifamycins (such as rifabutin), ritonavir, St. John's wort, drugs used to treat seizures (such as carbamazepine, phenytoin), among others.Aspirin can increase the risk of bleeding when used with this medication. However, if your doctor has told you to take low-dose aspirin to prevent heart attack or stroke (usually 81-162 milligrams a day), you should keep taking the aspirin unless your doctor tells you not to. Ask your doctor or pharmacist for more details.
OVERDOSE:
If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call 1-800-222-1222. Canada residents can call 1-844-764-7669. Symptoms of overdose may include: bloody/black/tarry stools, pink/dark urine, unusual/prolonged bleeding.
NOTES:
Do not share this medication with others.Lab and/or medical tests (such as hematocrit/hemoglobin, red blood cell count) may be done while you are taking this medication. Keep all medical and lab appointments. Consult your doctor for more details.
MISSED DOSE:
If you miss a dose, take it as soon as you remember. If it is near the time of the next dose, skip the missed dose. Take your next dose at the regular time. Do not double the dose to catch up.
STORAGE:
Store at room temperature away from light and moisture. Do not store in the bathroom. If you are crushing the tablet and mixing it as directed in water, apple juice, or applesauce, use the mixture within 4 hours of preparing it. Keep all medications away from children and pets.Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
MEDICAL ALERT: Your condition can cause complications in a medical emergency. For information about enrolling in MedicAlert, call 1-888-633-4298 (US) or 1-800-668-1507 (Canada).
Information last revised May 2024. Copyright(c) 2024 First Databank, Inc.
IMPORTANT: HOW TO USE THIS INFORMATION: This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you. This information is not individual medical advice and does not substitute for the advice of your health care professional. Always ask your health care professional for complete information about this product and your specific health needs.
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: