- Anti-hestaminic & Respiratory Drugs (20)
- Anti-inflammatory Drugs (197) +-
- Baby & Mom (1339) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (52)
- Beauty (3118) +-
- Beauty > Skin Care > whitening (311)
- Chemotherapy & Immune Response (885) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (11)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (290)
- CIRCULATORY DISTURBANCE AGENTS (24)
- Diet & Fitness Products (284) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (191)
- HEMATOLOGY (39)
-
Medical Supplies (506)
+-
- Chemicals & Disinfectants (19)
- Dental Supplies (31)
- Devices & Instruments (11)
- Diabetic Supplies (121)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- KIDNEY UNIT SUPPLIES (21)
- Lab Supplies (3)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (2)
- Sanitary (5)
- Sterilization Supplies (1)
- Surgical Sutures (4)
- Syringes (3)
-
Medicines & Health (2723)
+-
- Allergy & Sinus (95)
- Children's Health Care (54)
- Cough, Cold & Flu (281)
- Digestive Health & Nausea (231)
- Ear, Nose & Throat Care (179)
- Eye Care (124)
- Feminine Care (323)
- Foot Care (12)
- Orthopaedic Appliances (1)
- Pain Relief & Management (244)
- Pill Organizer (2)
- Skin Treatments (865)
- Sleep & Snoring Aids (2)
- Support & Braces (8)
- Medicines & health > Gout releif (42)
- Natural & Organic Products (81) +-
- OTC > Analgesics > Anti-inflammatory Drugs (44)
-
Personal Care (3343)
+-
- Bath & Body (271)
- Deodorant & Anti-perspirants (191)
- Ear, Nose & Throat Care (175)
- Eye Care (131)
- Feminine Care (372)
- Foot Care (20)
- Hair Care (500)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (26)
- Massage & Relaxation (17)
- Natural & Organic Personal Care (7)
- Oral Care (91)
- Pregnancy & Fertility (64)
- Shaving & Grooming (75)
- Sun Care (80)
-
Prescription Drugs (2932)
+-
- Analgesics (184)
- Cardiovascular System (374)
- Drugs Affecting Musculoskeletal System (65)
- Drugs Used In Infections (56)
- Ear & Nose Drugs (2)
- Endocrine System (177)
- Gastrointestinal Tract (243)
- Gastrointestinal Tract > Hepatology > Liver treatment (60)
- GYNECOLOGY (2)
- Miscellaneous (11)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (47)
- NEUROLOGY (228)
- Nutrients & Blood Electrolytes (2)
- Respiratory System (154)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (115)
- Vaccines (1)
- Prescription drugs > Cardiovascular system > Anti-hypertension drugs (242)
- Sexual Wellness (304) +-
- Vitamins & Minerals Supplements (1225) +-
Ex Tax: 14,160EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

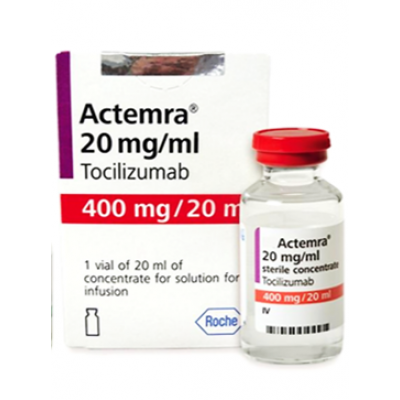
ACTEMRA 400 MG / 20 ML ( TOCILIZUMAB 20 ML / ML ) CONCENTRATE FOR DILUTION FOR IV INFUSION VIAL
IMPORTANT SAFETY INFORMATION:
ACTEMRA can cause serious side effects
Serious Infections
ACTEMRA changes the way your immune system works. This can make you more likely to get infections or make any current infection worse. Some people have serious infections while taking ACTEMRA, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have died from these infections. Your healthcare provider should assess you for TB before starting, during and after treatment with ACTEMRA (except if you have COVID-19).
Before starting ACTEMRA, tell your healthcare provider if you have:
- an infection, think you may have an infection, are being treated for an infection, or get a lot of infections that return. Symptoms of an infection, with or without a fever, include sweating or chills; shortness of breath; warm, red or painful skin or sores on your body; feeling very tired; muscle aches; blood in phlegm; diarrhea or stomach pain; cough; weight loss; burning when you urinate or urinating more than normal
- any of the following conditions that may give you a higher chance of getting infections: diabetes, HIV, or a weak immune system
- tuberculosis (TB), or have been in close contact with someone with TB
- live or have lived, or have traveled to certain parts of the United States where there is an increased chance of getting fungal infections. These parts include the Ohio and Mississippi River valleys and the Southwest
- hepatitis B or have had hepatitis B
If you have COVID-19, your healthcare provider should monitor you for signs and symptoms of new infections during and after treatment with ACTEMRA.
Who should not take ACTEMRA?
Do not take ACTEMRA if you are allergic to tocilizumab, or any of the ingredients in ACTEMRA.
Be sure to talk to your healthcare provider if you see any signs of these serious side effects:
Tears (perforation) of the Stomach or Intestines
If you have diverticulitis (inflammation in parts of the large intestine), talk to your healthcare provider before taking ACTEMRA. Some people taking ACTEMRA may develop a hole in the wall of their stomach or intestines (also known as a perforation). This happens most often in people who also take nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or methotrexate.
Tell your healthcare provider right away if you see any of these side effects: fever, new onset stomach-area pain that does not go away, or if you see a change in your bowel habits.
Liver problems (Hepatotoxicity)
Some people have experienced serious life-threatening liver problems, which required liver transplant or led to death. Your healthcare provider may tell you to stop taking ACTEMRA if you develop new or worsening liver problems during treatment with ACTEMRA. Tell your healthcare provider right away if you have any of the following symptoms:
- feeling tired (fatigue)
- lack of appetite for several days or longer (anorexia)
- yellowing of your skin or the whites of your eyes (jaundice)
- abdominal swelling and pain on the right side of the stomach-area
- light colored stools
- weakness
- nausea and vomiting
- confusion
- dark “tea-colored” urine
Changes in Blood Test Results
Your healthcare provider should do blood tests before you start receiving ACTEMRA. If you have rheumatoid arthritis (RA) or giant cell arteritis (GCA), or systemic sclerosis-interstitial lung disease (SSc-ILD) your healthcare provider should do blood tests 4 to 8 weeks after you start receiving ACTEMRA for the first 6 months and then every 3 months after that. If you have polyarticular juvenile idiopathic arthritis (PJIA) you will have blood tests done every 4 to 8 weeks during treatment. If you have systemic juvenile idiopathic arthritis (SJIA) you will have blood tests done every 2 to 4 weeks during treatment. These blood tests are to check for the following side effects of ACTEMRA:
- Low neutrophil count: neutrophils are white blood cells that help the body fight infection
- Low platelet count: platelets are blood cells that help with clotting, which stops bleeding
- Increase in liver function test levels
- Increase in blood cholesterol levels: your cholesterol levels should be checked 4 to 8 weeks after you start receiving ACTEMRA.
Your healthcare provider will determine how often you will have follow-up blood tests. Make sure you get all your follow-up blood tests done as ordered by your healthcare provider.
You should not receive ACTEMRA if your neutrophil and platelet counts are too low or your liver function test levels are too high. Changes in blood test results may cause your healthcare provider to stop your ACTEMRA treatment for a time or change your dose.
Cancer
ACTEMRA may increase your risk of certain cancers by changing the way your immune system works.
Hepatitis B Infection
If you have or are a carrier of the hepatitis B virus (a virus that affects the liver), the virus may become active while you use ACTEMRA. Your healthcare provider may do blood tests before you start treatment with ACTEMRA and while you are using ACTEMRA. Tell your healthcare provider if you have any signs of these symptoms:
- feel very tired
- skin or eyes look yellow
- little or no appetite
- vomiting
- clay-colored bowel movements
- fevers
- chills
- stomach discomfort
- muscle aches
- dark urine
- skin rash
Serious Allergic Reactions
Serious allergic reactions, including death, can happen with ACTEMRA. These reactions can happen with any infusion or injection of ACTEMRA, even if they did not occur with an earlier infusion or injection. Stop taking ACTEMRA, contact your healthcare provider, and get emergency help right away if you have any of the following signs of a serious allergic reaction:
- swelling of the face, lips, mouth, or tongue
- trouble breathing
- wheezing
- severe itching
- skin rash, hives, redness, or swelling outside of the injection site area
- dizziness or fainting
- fast heartbeat or pounding in your chest (tachycardia)
- sweating
Nervous System Problems
While rare, Multiple Sclerosis has been diagnosed in people who take ACTEMRA. It is not known what effect ACTEMRA may have on some nervous system disorders.
What should I tell my healthcare provider before receiving ACTEMRA?
ACTEMRA may not be right for you. Before receiving ACTEMRA, tell your healthcare provider if you:
- have an infection
- have liver problems
- have any stomach-area (abdominal) pain or been diagnosed with diverticulitis or ulcers in your stomach or intestines
- have had a reaction to tocilizumab or any of the ingredients in ACTEMRA before
- have or had a condition that affects your nervous system, such as multiple sclerosis
- have recently received or are scheduled to receive a vaccine
- plan to have surgery or a medical procedure
- have any other medical conditions
- plan to become pregnant or are pregnant. It is not known if ACTEMRA will harm your unborn baby.
- plan to breast-feed or are breast-feeding. You and your healthcare provider should decide if you will take ACTEMRA or breast-feed. You should not do both.
- are taking any medications, including prescription and nonprescription medicines, vitamins, and herbal supplements.
The most common side effects of ACTEMRA include:
- upper respiratory tract infections (common cold, sinus infections)
- headache
- increased blood pressure (hypertension)
- injection site reactions
Tell your healthcare provider about any side effect that bothers you or does not go away. These are not all the possible side effects of ACTEMRA.
ACTEMRA & Pregnancy
Tell your healthcare provider if you are planning to become pregnant, are pregnant, plan to breast-feed, or are breast-feeding. You and your healthcare provider should decide if you will take ACTEMRA or breast-feed. You should not do both.
Tell your healthcare provider if you have any side effects. You may report side effects to the FDA at 1-800-FDA-1088. You may also report side effects to Genentech at 1-888-835-2555.
Please see full Prescribing Information and the Medication Guide, including Serious Side Effects, for more Important Safety Information.
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: