- Anti-hestaminic & Respiratory Drugs (20)
- Anti-inflammatory Drugs (197) +-
- Baby & Mom (1346) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (52)
- Beauty (3129) +-
- Beauty > Skin Care > whitening (309)
- Chemotherapy & Immune Response (885) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (11)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (290)
- CIRCULATORY DISTURBANCE AGENTS (24)
- Diet & Fitness Products (284) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (191)
- HEMATOLOGY (39)
-
Medical Supplies (506)
+-
- Chemicals & Disinfectants (19)
- Dental Supplies (31)
- Devices & Instruments (11)
- Diabetic Supplies (121)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- KIDNEY UNIT SUPPLIES (21)
- Lab Supplies (3)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (2)
- Sanitary (5)
- Sterilization Supplies (1)
- Surgical Sutures (4)
- Syringes (3)
-
Medicines & Health (2727)
+-
- Allergy & Sinus (97)
- Children's Health Care (54)
- Cough, Cold & Flu (283)
- Digestive Health & Nausea (231)
- Ear, Nose & Throat Care (181)
- Eye Care (124)
- Feminine Care (323)
- Foot Care (12)
- Orthopaedic Appliances (1)
- Pain Relief & Management (244)
- Pill Organizer (2)
- Skin Treatments (863)
- Sleep & Snoring Aids (2)
- Support & Braces (8)
- Medicines & health > Gout releif (42)
- Natural & Organic Products (81) +-
- OTC > Analgesics > Anti-inflammatory Drugs (44)
-
Personal Care (3363)
+-
- Bath & Body (273)
- Deodorant & Anti-perspirants (191)
- Ear, Nose & Throat Care (177)
- Eye Care (131)
- Feminine Care (372)
- Foot Care (20)
- Hair Care (511)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (26)
- Massage & Relaxation (17)
- Natural & Organic Personal Care (7)
- Oral Care (91)
- Pregnancy & Fertility (64)
- Shaving & Grooming (75)
- Sun Care (80)
-
Prescription Drugs (2935)
+-
- Analgesics (184)
- Cardiovascular System (377)
- Drugs Affecting Musculoskeletal System (65)
- Drugs Used In Infections (56)
- Ear & Nose Drugs (2)
- Endocrine System (177)
- Gastrointestinal Tract (243)
- Gastrointestinal Tract > Hepatology > Liver treatment (60)
- GYNECOLOGY (2)
- Miscellaneous (11)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (47)
- NEUROLOGY (228)
- Nutrients & Blood Electrolytes (2)
- Respiratory System (154)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (115)
- Vaccines (1)
- Prescription drugs > Cardiovascular system > Anti-hypertension drugs (242)
- Sexual Wellness (304) +-
- Vitamins & Minerals Supplements (1230) +-
Ex Tax: 39EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

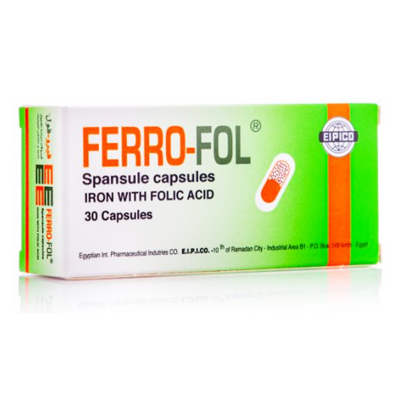
Ferrofol ( Dried ferrous sulphate 150 mg + Equivalent to Iron 47 mg + Folic acid 0.5 mg ) 30 capsules
Composition
Each spansule capsule contains:
Dried ferrous sulphate ......................................................... 150 mg
Equivalent to Iron .................................................................... 47 mg
Folic acid .................................................................................. 0.5 mg
Inactive ingredients:
Maize starch, talc purified, sucrose, calcium sulphate dehydrate, titanium dioxide, povidone, glyceryl monostearate, bees wax white, gelatin, heavy kaolin, FD&C Red No.3 lake, FD&C Yellow No.6 lake.
Therapeutic Indications
Prophylaxis of iron and folic acid deficiency during pregnancy and lactation.
Iron-deficiency anaemia.
Dosage and Administration
Adults Only:
One FERROFOL Capsule daily.
Iron-deficiency anaemia and some pregnant women may need a higher dose of iron because of dietary or other factors.
Contraindications
Hypersensitivity to any of the ingredients of FERROFOL.
FERROFOL should not be given to patients receiving repeated blood transfusions or to patients with anaemias not produced by iron deficiency unless iron deficiency is also present.
Warnings and Precautions
FERROFOL should never be given for the treatment of undiagnosed megaloblastic anaemia, since folic acid may produce a haematopoietic response in patients with a megaloblastic anaemia due to vitamin B12 deficiency without preventing aggravation of neurological symptoms, which may lead to serious neurological damage.
FERROFOL should be used with caution in patients with iron-storage or iron-absorption diseases such as haemochromatosis, haemoglobinopathies, or existing gastrointestinal diseases such as inflammatory bowel disease, intestinal strictures and diverticulae.
Drug Interactions
Concurrent use of iron with antacids, calcium supplements, tea, coffee, milk or milk products may decrease iron absorption and so iron supplements should not be taken within one hour before or two hours after ingestion of any of the above.
Concurrent administration of tetracyclines, trientine with iron salts may impair absorption of both agents, and so if treatment with both drugs is required, a time interval of about 2-3 hours should be allowed between them.
Concomitant administration of penicillamine with iron salts may decrease absorption of penicillamine.
Concomitant administration of folic acid with oral contraceptives, aminosalicylic may reduce absorption of folic acid.
Concomitant administration of folic acid with antiepileptic drugs such as phenytoin may reduce plasma concentration of these drugs.
Pregnancy and Lactation
FERROFOL can be used safely during pregnancy and lactation.
Undesirable Effects
FERROFOL is generally well–tolerated.
Side effects may include abdominal pain, GI irritation, nausea, vomiting, constipation, diarrhoea, darkening of the stool.
Overdose
It has been stated that more than the equivalent of 20 mg/kg of iron could lead to some symptoms of toxicity and that in a young child the equivalent of about 60 mg/kg of iron should be regarded as extremely dangerous.
Symptoms:
In the first phase, which occurs up to 6 hours after oral ingestion, gastrointestinal toxicity, notably vomiting and diarrhoea, predominates.
Other effects may include cardiovascular disorders such as hypotension, metabolic changes including acidosis and hyperglycaemia, and CNS depression ranging from lethargy to coma.
Patients with only mild to moderate poisoning do not generally progress past this first phase.
The second phase, which is not always seen, may occur at 6-24 hours after ingestion and is characterised by a temporary remission or clinical stabilisation.
In the third phase, 12-48 hours after ingestion, gastrointestinal toxicity recurs together with shock, metabolic acidosis, severe lethargy or coma, hepatic necrosis and jaundice, hypoglycaemia, coagulation disorders, oliguria or renal failure, and possible myocardial dysfunction.
The fourth phase may occur several weeks after ingestion and is characterised by gastrointestinal obstruction and possibly late hepatic damage.
Treatment:
Treatment includes gastric lavage followed by administration of 5 g desferrioxamine by IV infusion. Serum iron levels should be monitored and supportive and symptomatic measures should be applied.
Pharmacological Properties
FERROFOL is a haematinic preparation especially formulated for prophylaxis and treatment of iron and folic acid deficiency during pregnancy and lactation.
FERROFOL is formulated as spansule capsules to release most of the iron in the upper small intestine where absorption is greatest and not in the stomach where gastric irritation may occur.
Ferrous sulphate acts as a source of iron for iron-deficiency anaemia. Each spansule capsule contains 150 mg of dried ferrous sulphate; four-fifths of the iron is especially formulated for sustained release over a period of several hours.
Folic acid is a member of the vitamin B group; it is reduced in the body to tetrahydrofolate, which is a coenzyme for various metabolic processes including the synthesis of DNA.
Both iron and folic acid are essential for erythropoiesis and maturation of RBCs.
Storage
Store in a dry place at a temperature not exceeding 30°C.
FERROFOL Spansule Capsules: Box containing 3 strips of 10 spansule capsules each.
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: