- Anti-hestaminic & Respiratory Drugs (20)
- Anti-inflammatory Drugs (192) +-
- Baby & Mom (1314) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (52)
- Beauty (2907) +-
- Beauty > Skin Care > whitening (274)
- Chemotherapy & Immune Response (882) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (11)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (289)
- CIRCULATORY DISTURBANCE AGENTS (24)
- Diet & Fitness Products (280) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (191)
- HEMATOLOGY (39)
-
Medical Supplies (503)
+-
- Chemicals & Disinfectants (19)
- Dental Supplies (31)
- Devices & Instruments (10)
- Diabetic Supplies (121)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- KIDNEY UNIT SUPPLIES (21)
- Lab Supplies (3)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (2)
- Sanitary (5)
- Sterilization Supplies (0)
- Surgical Sutures (4)
- Syringes (3)
-
Medicines & Health (2601)
+-
- Allergy & Sinus (95)
- Children's Health Care (54)
- Cough, Cold & Flu (277)
- Digestive Health & Nausea (225)
- Ear, Nose & Throat Care (179)
- Eye Care (117)
- Feminine Care (321)
- Foot Care (4)
- Orthopaedic Appliances (0)
- Pain Relief & Management (237)
- Pill Organizer (2)
- Skin Treatments (784)
- Sleep & Snoring Aids (2)
- Support & Braces (7)
- Medicines & health > Gout releif (42)
- Natural & Organic Products (81) +-
- OTC > Analgesics > Anti-inflammatory Drugs (44)
-
Personal Care (3187)
+-
- Bath & Body (261)
- Deodorant & Anti-perspirants (182)
- Ear, Nose & Throat Care (175)
- Eye Care (123)
- Feminine Care (370)
- Foot Care (12)
- Hair Care (468)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (22)
- Massage & Relaxation (17)
- Natural & Organic Personal Care (7)
- Oral Care (88)
- Pregnancy & Fertility (64)
- Shaving & Grooming (65)
- Sun Care (78)
-
Prescription Drugs (2870)
+-
- Analgesics (181)
- Cardiovascular System (374)
- Drugs Affecting Musculoskeletal System (65)
- Drugs Used In Infections (56)
- Ear & Nose Drugs (2)
- Endocrine System (176)
- Gastrointestinal Tract (242)
- Gastrointestinal Tract > Hepatology > Liver treatment (61)
- GYNECOLOGY (2)
- Miscellaneous (11)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (46)
- NEUROLOGY (222)
- Nutrients & Blood Electrolytes (2)
- Respiratory System (154)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (68)
- Vaccines (1)
- Prescription drugs > Cardiovascular system > Anti-hypertension drugs (242)
- Sexual Wellness (301) +-
- Vitamins & Minerals Supplements (1197) +-
Ex Tax: 80EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

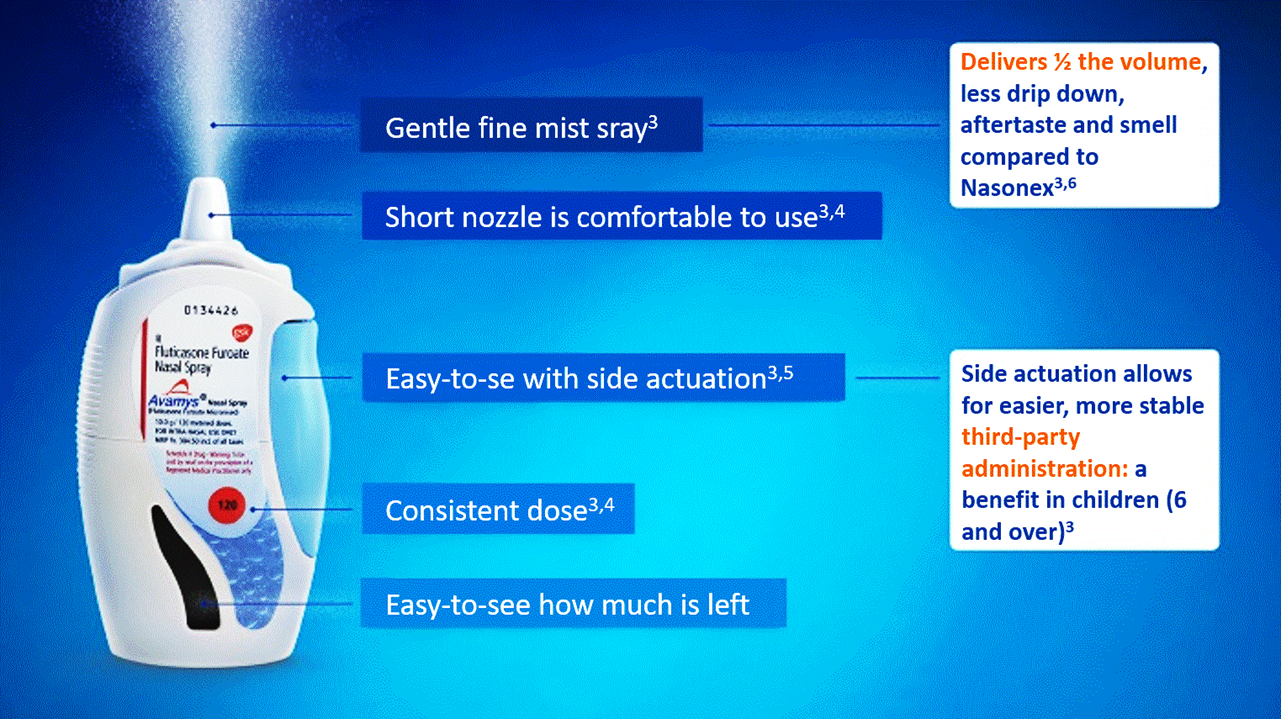
Avamys ® Nasal Spray Suspension ( Fluticasone furoate 27.5 micrograms / metered spray ) 120 doses
Avamys nasal spray is indicated for the treatment of the symptoms of allergic rhinitis in adults, adolescents and children (aged six and over).
Indication
Avamys nasal spray is indicated for the treatment of the symptoms of allergic rhinitis 1
Avamys is indicated in adults, adolescents and children (6 years and over)
Avamys (fluticasone furoate) Prescribing Information
(Please refer to the full Summary of Product Characteristics before prescribing)
Content Lab code: PI-4870 April 2020
Avamys® Nasal Spray Suspension (fluticasone furoate
27.5 micrograms/metered spray)
Uses: Treatment of symptoms of allergic rhinitis in
adults, adolescents and children aged 6 years and over.
Dosage and Administration: For intranasal use only.
Adults and adolescents (12 years and older): Two sprays
per nostril once daily (total daily dose, 110 micrograms).
Once symptoms controlled, use maintenance dose of
one spray per nostril once daily (total daily dose, 55
micrograms). Reduce to lowest dose at which effective
control of symptoms is maintained. Children aged 6 to
11 years: One spray per nostril once daily (total daily
dose, 55 micrograms). If patient is not adequately
responding, increase daily dose to 110 micrograms (two
sprays per nostril, once daily) and reduce back down to
55 micrograms daily dose once control is achieved.
The duration of treatment should be restricted to the period
that corresponds to allergenic exposure.
Contraindications: Hypersensitivity to active substance
or excipients. Special warnings and precautions:
Systemic effects of nasal corticosteroids may occur,
particularly when prescribed at high doses for prolonged
periods. These effects are much less likely to occur than
with oral corticosteroids and may vary in individual
patients and between different corticosteroid
preparations.
Potential systemic effects may include
Cushing’s syndrome, Cushingoid features, adrenal
suppression, growth retardation in children and
adolescents, cataract, glaucoma and more rarely, a
range of psychological or behavioural effects including
psychomotor hyperactivity, sleep disorders, anxiety,
depression or aggression (particularly in children). If a
patient presents with blurred vision or other visual
disturbance, consider referral to an ophthalmologist for
evaluation. Causes may include cataract, glaucoma or
rare diseases such as central serous chorioretinopathy
(CSCR) which have been reported after use of systemic
and topical corticosteroids.
Treatment with higher than
recommended doses of nasal corticosteroids may result
in clinically significant adrenal suppression. If there is
evidence for higher than recommended doses being
used, then additional systemic corticosteroid cover
should be considered during periods of stress or elective
surgery. It is recommended that growth of children
receiving prolonged treatment with nasal corticosteroids
is regularly monitored. Aim to reduce to the lowest
effective dose and consider referring to a paediatric
specialist.
Due to risk of increased systemic exposure,
caution is advised when prescribing concurrently with
other corticosteroids.
Drug interactions: Caution is
recommended when co-administering fluticasone
furoate with potent CYP3A4 inhibitors
(e.g. ketoconazole) including cobicistat-containing
products as an increase in the risk of systemic side
effects is expected. Co-administration with ritonavir is
not recommended because of the risk of increased
systemic exposure of fluticasone furoate. Coadministration should be avoided unless the benefit
outweighs the increased risk of systemic corticosteroid
side effects, in which case patients should be monitored
for systemic corticosteroid side effects.
Pregnancy lactation and fertility: No adequate data available.
Recommended nasal doses result in minimal systemic
exposure. It is unknown if fluticasone furoate nasal
spray is excreted in breast milk. Only use during
pregnancy or in breastfeeding women if the expected
benefits to the mother outweigh the possible risks to
the foetus or child. No fertility data in humans.
Undesirable effects: Very common (≥1/10): epistaxis.
Epistaxis was generally mild to moderate, with
incidences in adults and adolescents higher in longerterm use (more than 6 weeks). Common (≥1/100 and
<1/10): headache, nasal ulceration. Uncommon
(≥1/1000 and <1/100): rhinalgia, nasal discomfort, nasal
dryness. Rare (≥1/10,000 and <1/1000): hypersensitivity
reactions including anaphylaxis, angioedema, rash, and
urticaria. Very rare (<1/10,000): Nasal septum
perforation. Not known: Bronchospasm, dyspnoea,
transient ocular changes – blurred vision, growth
retardation Consult the SPC in relation to other adverse
reactions.
Presentation and Basic NHS cost: Avamys
Nasal Spray Suspension: 120 sprays: £6.44 Marketing
Authorisation Number: EU/1/07/434/001-003. Legal
category: POM. PL holder: GlaxoSmithKline (Ireland)
Limited, 12 Riverwalk, Citywest Business Campus, Dublin
24, Ireland. Content Lab code: PI-4870 Last date of
revision: April 2020
Avamys is a registered trade mark of the GSK group of
companies.
| Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221 441. |

Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: