- Anti-hestaminic & Respiratory Drugs (20)
- Anti-inflammatory Drugs (197) +-
- Baby & Mom (1346) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (52)
- Beauty (3129) +-
- Beauty > Skin Care > whitening (309)
- Chemotherapy & Immune Response (885) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (11)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (290)
- CIRCULATORY DISTURBANCE AGENTS (24)
- Diet & Fitness Products (284) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (191)
- HEMATOLOGY (39)
-
Medical Supplies (506)
+-
- Chemicals & Disinfectants (19)
- Dental Supplies (31)
- Devices & Instruments (11)
- Diabetic Supplies (121)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- KIDNEY UNIT SUPPLIES (21)
- Lab Supplies (3)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (2)
- Sanitary (5)
- Sterilization Supplies (1)
- Surgical Sutures (4)
- Syringes (3)
-
Medicines & Health (2727)
+-
- Allergy & Sinus (97)
- Children's Health Care (54)
- Cough, Cold & Flu (283)
- Digestive Health & Nausea (231)
- Ear, Nose & Throat Care (181)
- Eye Care (124)
- Feminine Care (323)
- Foot Care (12)
- Orthopaedic Appliances (1)
- Pain Relief & Management (244)
- Pill Organizer (2)
- Skin Treatments (863)
- Sleep & Snoring Aids (2)
- Support & Braces (8)
- Medicines & health > Gout releif (42)
- Natural & Organic Products (81) +-
- OTC > Analgesics > Anti-inflammatory Drugs (44)
-
Personal Care (3363)
+-
- Bath & Body (273)
- Deodorant & Anti-perspirants (191)
- Ear, Nose & Throat Care (177)
- Eye Care (131)
- Feminine Care (372)
- Foot Care (20)
- Hair Care (511)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (26)
- Massage & Relaxation (17)
- Natural & Organic Personal Care (7)
- Oral Care (91)
- Pregnancy & Fertility (64)
- Shaving & Grooming (75)
- Sun Care (80)
-
Prescription Drugs (2935)
+-
- Analgesics (184)
- Cardiovascular System (377)
- Drugs Affecting Musculoskeletal System (65)
- Drugs Used In Infections (56)
- Ear & Nose Drugs (2)
- Endocrine System (177)
- Gastrointestinal Tract (243)
- Gastrointestinal Tract > Hepatology > Liver treatment (60)
- GYNECOLOGY (2)
- Miscellaneous (11)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (47)
- NEUROLOGY (228)
- Nutrients & Blood Electrolytes (2)
- Respiratory System (154)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (115)
- Vaccines (1)
- Prescription drugs > Cardiovascular system > Anti-hypertension drugs (242)
- Sexual Wellness (304) +-
- Vitamins & Minerals Supplements (1230) +-
Ex Tax: 1,075EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

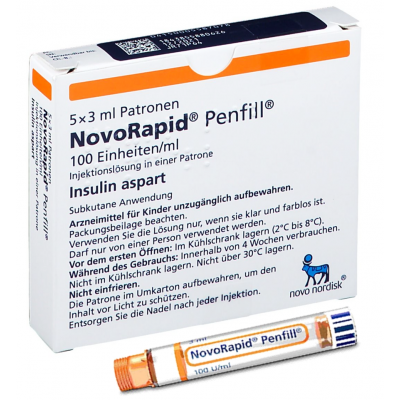
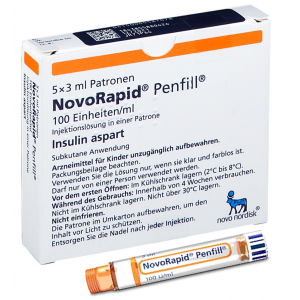
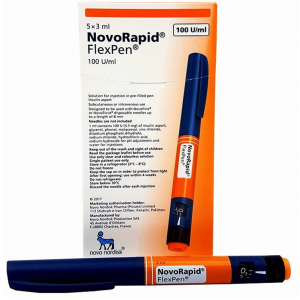
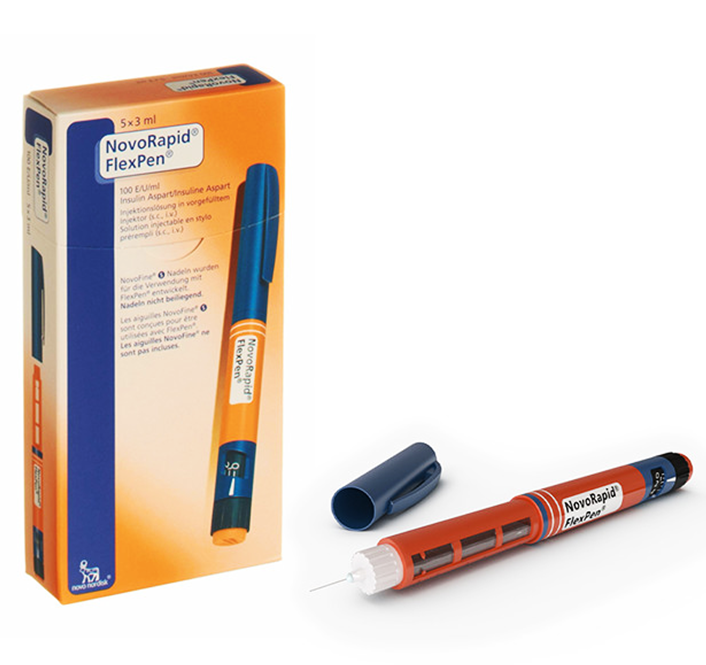
NovoRapid PenFill 100 units / ml ( insulin aspart ) 5 PenFills



1. Name of the medicinal product
NovoRapid 100 units/ml solution for injection in vial
NovoRapid Penfill 100 units/ml solution for injection in cartridge
NovoRapid FlexPen 100 units/ml solution for injection in pre-filled pen
NovoRapid FlexTouch 100 units/ml solution for injection in pre-filled pen
NovoRapid PumpCart 100 units/ml solution for injection in cartridge
2. Qualitative and quantitative composition
NovoRapid vial
1 vial contains 10 ml equivalent to 1,000 units.1 ml solution contains 100 units insulin aspart* (equivalent to 3.5 mg).
NovoRapid Penfill
1 cartridge contains 3 ml equivalent to 300 units.1 ml solution contains 100 units insulin aspart* (equivalent to 3.5 mg).
NovoRapid FlexPen/NovoRapid FlexTouch
1 pre-filled pen contains 3 ml equivalent to 300 units.1 ml solution contains 100 units insulin aspart* (equivalent to 3.5 mg).
NovoRapid PumpCart
1 cartridge contains 1.6 ml equivalent to 160 units.1 ml solution contains 100 units insulin aspart* (equivalent to 3.5 mg).
*Insulin aspart is produced in Saccharomyces cerevisiae by recombinant DNA technology.
For the full list of excipients, see section 6.1.
3. Pharmaceutical form
Solution for injection.
The solution is clear, colourless and aqueous.
4. Clinical particulars
4.1 Therapeutic indications
NovoRapid is indicated for treatment of diabetes mellitus in adults, adolescents and children aged 1 year and above.
4.2 Posology and method of administration
Posology
The potency of insulin analogues, including insulin aspart, is expressed in units, whereas the potency of human insulin is expressed in international units.
NovoRapid dosing is individual and determined in accordance with the needs of the patient. It should normally be used in combination with intermediate-acting or long-acting insulin.
Moreover NovoRapid vial and NovoRapid PumpCart can be used for continuous subcutaneous insulin infusion (CSII) in pump systems.
NovoRapid vial can also be used if intravenous administration of insulin aspart, by physicians or other healthcare staff, is applicable.
Blood glucose monitoring and insulin dose adjustments are recommended to achieve optimal glycaemic control.
The individual insulin requirement in adults and children is usually between 0.5 and 1.0 unit/kg/day. In a basal-bolus treatment regimen 50–70% of this requirement may be provided by NovoRapid and the remainder by intermediate-acting or long-acting insulin.
Adjustment of dose may be necessary if patients undertake increased physical activity, change their usual diet or during concomitant illness.
Special populations
Elderly (≥ 65 years old)
NovoRapid can be used in elderly patients.
In elderly patients, glucose monitoring should be intensified and the insulin aspart dose adjusted on an individual basis.
Renal and hepatic impairment
Renal or hepatic impairment may reduce the patient's insulin requirements.
In patients with renal or hepatic impairment, glucose monitoring should be intensified and the insulin aspart dose adjusted on an individual basis.
Paediatric population
NovoRapid can be used in children and adolescents aged 1 year and above in preference to soluble human insulin when a rapid onset of action might be beneficial, for example, in the timing of the injections in relation to meals (see sections 5.1 and 5.2).
The safety and efficacy of NovoRapid in children below 1 year of age have not been established.
No data are available.
Transfer from other insulin medicinal products
When transferring from other insulin medicinal products, adjustment of the NovoRapid dose and the dose of the basal insulin may be necessary. NovoRapid has a faster onset and a shorter duration of action than soluble human insulin. When injected subcutaneously into the abdominal wall, the onset of action will occur within 10–20 minutes of injection. The maximum effect is exerted between 1 and 3 hours after the injection. The duration of action is 3 to 5 hours.
Close glucose monitoring is recommended during the transfer and in the initial weeks thereafter (see section 4.4).
Method of administration
NovoRapid is a rapid-acting insulin analogue.
NovoRapid is administered subcutaneously by injection in the abdominal wall, the thigh, the upper arm, the deltoid region or the gluteal region. Injection sites should always be rotated within the same region in order to reduce the risk of lipodystrophy. Subcutaneous injection in the abdominal wall ensures a faster absorption than other injection sites. Compared to soluble human insulin the faster onset of action of NovoRapid is maintained regardless of the injection site. The duration of action will vary according to the dose, injection site, blood flow, temperature and level of physical activity.
Due to the faster onset of action, NovoRapid should generally be given immediately before a meal. When necessary NovoRapid can be given soon after a meal.
NovoRapid vial/NovoRapid PumpCart
Continuous Subcutaneous Insulin Infusion (CSII)
NovoRapid may be used for CSII in pump systems suitable for insulin infusion. CSII should be administered in the abdominal wall. Infusion sites should be rotated.
When used with an insulin infusion pump, NovoRapid should not be mixed with any other insulin medicinal products.
Patients using CSII should be comprehensively instructed in the use of the pump system and use the correct reservoir and tubing for the pump (see section 6.6). The infusion set (tubing and cannula) should be changed in accordance with the instructions in the product information supplied with the infusion set.
Patients administering NovoRapid by CSII must have an alternative insulin delivery method available in case of pump system failure.
NovoRapid vial
Intravenous use
If necessary, NovoRapid can be administered intravenously which should be carried out by physicians or other healthcare staff.
For intravenous use, infusion systems with NovoRapid 100 units/ml at concentrations from 0.05 unit/ml to 1.0 unit/ml insulin aspart in the infusion fluids 0.9% sodium chloride, 5% dextrose or 10% dextrose including 40 mmol/l potassium chloride using polypropylene infusion bags, are stable at room temperature for 24 hours.
Although stable over time, a certain amount of insulin will be initially adsorbed to the material of the infusion bag. Monitoring of blood glucose is necessary during insulin infusion.
Mixing two types of insulins
NovoRapid can only be mixed with NPH (Neutral Protamine Hagedorn) insulin in a syringe for subcutaneous use. When NovoRapid is mixed with NPH insulin, NovoRapid should be drawn into the syringe first, and the mixture should be injected immediately after mixing. Insulin mixtures should not be administered intravenously or used with a subcutaneous insulin infusion pump.
Administration with a syringe
NovoRapid vials are for use with insulin syringes with the corresponding unit scale. See also section 6.2.
NovoRapid Penfill
Administration with an insulin delivery system
NovoRapid Penfill is designed to be used with Novo Nordisk insulin delivery systems and NovoFine or NovoTwist needles. NovoRapid Penfill is only suitable for subcutaneous injections from a reusable pen. If administration by syringe or intravenous injection is necessary, a vial should be used. If administration by infusion pump is necessary, a vial or NovoRapid PumpCart should be used.
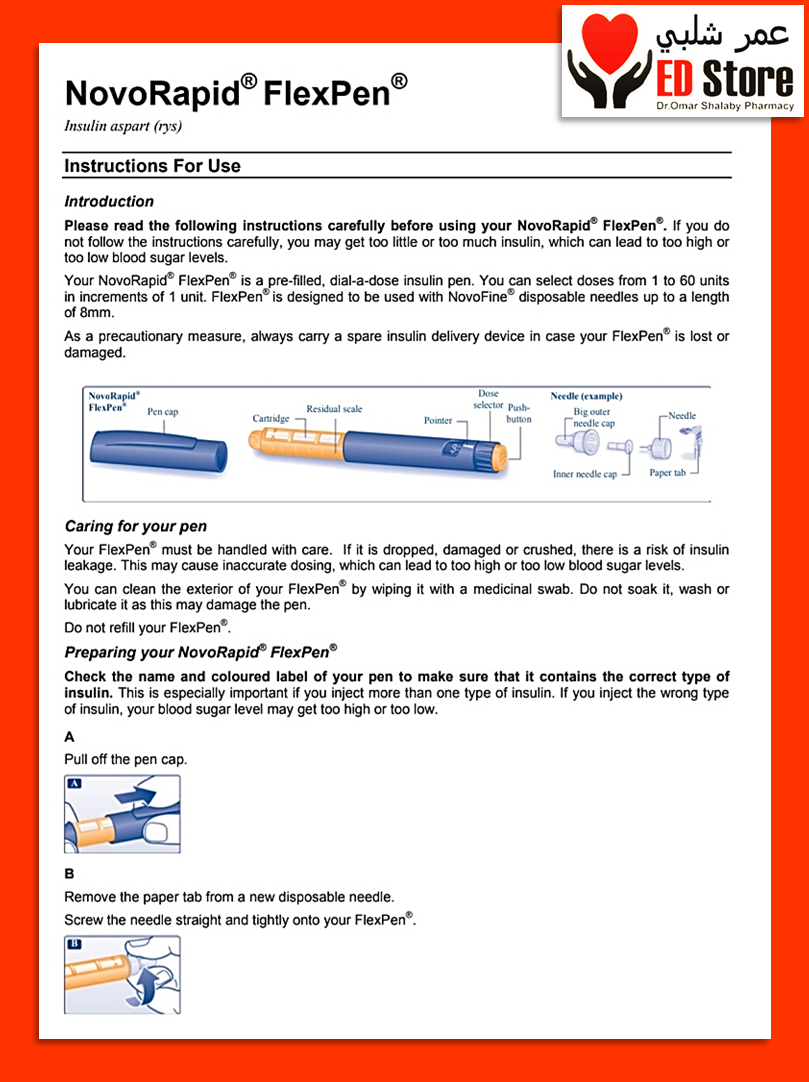
NovoRapid FlexPen
Administration with FlexPen
NovoRapid FlexPen is a pre-filled pen (colour-coded) designed to be used with NovoFine or NovoTwist disposable needles up to a length of 8 mm. FlexPen delivers 1–60 units in increments of 1 unit. NovoRapid FlexPen is only suitable for subcutaneous injections. If administration by syringe or intravenous injection is necessary, a vial should be used. If administration by infusion pump is necessary, a vial or NovoRapid PumpCart should be used.
NovoRapid FlexTouch
Administration with FlexTouch
NovoRapid FlexTouch is a pre-filled pen (colour-coded) designed to be used with NovoFine or NovoTwist disposable needles up to a length of 8 mm. FlexTouch delivers 1–80 units in increments of 1 unit. NovoRapid FlexTouch is only suitable for subcutaneous injections. If administration by syringe or intravenous injection is necessary, a vial should be used. If administration by infusion pump is necessary, a vial or NovoRapid PumpCart should be used.
NovoRapid PumpCart
Administration via Continuous Subcutaneous Insulin Infusion (CSII)
NovoRapid PumpCart is only for use with an insulin infusion pump system designed to be used with this cartridge, such as the Accu-Chek Insight and YpsoPump insulin pumps.
CSII should be administered in the abdominal wall. Infusion sites should be rotated. NovoRapid PumpCart is only suitable for CSII in pump systems suitable for insulin infusion. If administration by syringe or intravenous injection is necessary, a vial should be used.
For detailed user instructions, please refer to the package leaflet.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients (see section 6.1).
4.4 Special warnings and precautions for use
Before travelling between different time zones, the patient should seek the doctor's advice since this may mean that the patient has to take the insulin and meals at different times.
NovoRapid PumpCart
Misuse of NovoRapid PumpCart
NovoRapid PumpCart is only for use with an insulin infusion pump system designed to be used with this cartridge, such as the Accu-Chek Insight and YpsoPump insulin pumps. It must not be used with other devices not designed for NovoRapid PumpCart, as this may result in incorrect insulin dosing and subsequent hyper- or hypoglycaemia.
Hyperglycaemia
Inadequate dosing or discontinuation of treatment, especially in type 1 diabetes, may lead to hyperglycaemia and diabetic ketoacidosis. Usually the first symptoms of hyperglycaemia develop gradually over a period of hours or days. They include thirst, increased frequency of urination, nausea, vomiting, drowsiness, flushed dry skin, dry mouth, loss of appetite as well as acetone odour of breath. In type 1 diabetes, untreated hyperglycaemic events eventually lead to diabetic ketoacidosis, which is potentially lethal.
Hypoglycaemia
Omission of a meal or unplanned, strenuous physical exercise may lead to hypoglycaemia.
Especially in children, care should be taken to match insulin doses (especially in basal-bolus regimens) with food intake, physical activities and current blood glucose level in order to minimise the risk of hypoglycaemia.
Hypoglycaemia may occur if the insulin dose is too high in relation to the insulin requirement. In case of hypoglycaemia or if hypoglycaemia is suspected NovoRapid must not be injected. After stabilisation of patient's blood glucose adjustment of the dose should be considered (see sections 4.8 and 4.9).
Patients whose blood glucose control is greatly improved, e.g. by intensified insulin therapy, may experience a change in their usual warning symptoms of hypoglycaemia, and should be advised accordingly. Usual warning symptoms may disappear in patients with longstanding diabetes.
A consequence of the pharmacodynamics of rapid-acting insulin analogues is that if hypoglycaemia occurs, it may occur earlier after an injection when compared with soluble human insulin.
Since NovoRapid should be administered in immediate relation to a meal, the rapid onset of action should be considered in patients with concomitant diseases or treatment where a delayed absorption of food might be expected.
Concomitant illness, especially infections and feverish conditions, usually increases the patient's insulin requirements. Concomitant diseases in the kidney, liver or affecting the adrenal, pituitary or thyroid gland can require changes in the insulin dose.
When patients are transferred between different types of insulin medicinal products, the early warning symptoms of hypoglycaemia may change or become less pronounced than those experienced with their previous insulin.
Transfer from other insulin medicinal products
Transferring a patient to another type or brand of insulin should be done under strict medical supervision. Changes in strength, brand (manufacturer), type, origin (animal, human insulin or human insulin analogue) and/or method of manufacture (recombinant DNA versus animal source insulin) may result in the need for a change in dose. Patients transferred to NovoRapid from another type of insulin may require an increased number of daily injections or a change in dose from that used with their usual insulin medicinal products. If an adjustment is needed, it may occur with the first dose or during the first few weeks or months.
Injection site reactions
As with any insulin therapy, injection site reactions may occur and include pain, redness, hives, inflammation, bruising, swelling and itching. Continuous rotation of the injection site within a given area reduces the risk of developing these reactions. Reactions usually resolve in a few days to a few weeks. On rare occasions, injection site reactions may require discontinuation of NovoRapid.
Combination of NovoRapid with pioglitazone
Cases of cardiac failure have been reported when pioglitazone was used in combination with insulin, especially in patients with risk factors for development of cardiac heart failure. This should be kept in mind if treatment with the combination of pioglitazone and NovoRapid is considered. If the combination is used, patients should be observed for signs and symptoms of heart failure, weight gain and oedema. Pioglitazone should be discontinued if any deterioration in cardiac symptoms occurs.
Avoidance of accidental mix-ups/medication errors
Patients must be instructed to always check the insulin label before each injection to avoid accidental mix-ups between NovoRapid and other insulin products.
Insulin antibodies
Insulin administration may cause insulin antibodies to form. In rare cases, the presence of such insulin antibodies may necessitate adjustment of the insulin dose in order to correct a tendency to hyper- or hypoglycaemia.
4.5 Interaction with other medicinal products and other forms of interaction
A number of medicinal products are known to interact with the glucose metabolism.
The following substances may reduce the patient's insulin requirements:
Oral antidiabetic medicinal products, monoamine oxidase inhibitors (MAOI), beta-blockers, angiotensin converting enzyme (ACE) inhibitors, salicylates, anabolic steroids and sulphonamides.
The following substances may increase the patient's insulin requirements:
Oral contraceptives, thiazides, glucocorticoids, thyroid hormones, sympathomimetics, growth hormone and danazol.
Beta-blockers may mask the symptoms of hypoglycaemia.
Octreotide/lanreotide may either increase or decrease the insulin requirement.
Alcohol may intensify or reduce the hypoglycaemic effect of insulin.
4.6 Fertility, pregnancy and lactation
Pregnancy
NovoRapid (insulin aspart) can be used in pregnancy. Data from two randomised controlled clinical trials (322 and 27 exposed pregnancies) do not indicate any adverse effect of insulin aspart on pregnancy or on the health of the foetus/newborn when compared to human insulin (see section 5.1).
Intensified blood glucose control and monitoring of pregnant women with diabetes (type 1 diabetes, type 2 diabetes or gestational diabetes) are recommended throughout pregnancy and when contemplating pregnancy. Insulin requirements usually fall in the first trimester and increase subsequently during the second and third trimester. After delivery, insulin requirements normally return rapidly to pre-pregnancy values.
Breast-feeding
There are no restrictions on treatment with NovoRapid during breast-feeding. Insulin treatment of the nursing mother presents no risk to the baby. However, the NovoRapid dose may need to be adjusted.
Fertility
Animal reproduction studies have not revealed any differences between insulin aspart and human insulin regarding fertility.
4.7 Effects on ability to drive and use machines
The patient's ability to concentrate and react may be impaired as a result of hypoglycaemia. This may constitute a risk in situations where these abilities are of special importance (e.g. driving a car or operating machinery).
Patients should be advised to take precautions to avoid hypoglycaemia while driving. This is particularly important in those who have reduced or absent awareness of the warning signs of hypoglycaemia or have frequent episodes of hypoglycaemia. The advisability of driving should be considered in these circumstances.
4.8 Undesirable effects
Summary of the safety profile
Adverse reactions observed in patients using NovoRapid are mainly due to the pharmacologic effect of insulin.
The most frequently reported adverse reaction during treatment is hypoglycaemia. The frequencies of hypoglycaemia vary with patient population, dose regimens and level of glycaemic control (see section 4.8, Description of selected adverse reactions).
At the beginning of the insulin treatment, refraction anomalies, oedema and injection site reactions (pain, redness, hives, inflammation, bruising, swelling and itching at the injection site) may occur. These reactions are usually of transitory nature. Fast improvement in blood glucose control may be associated with acute painful neuropathy, which is usually reversible. Intensification of insulin therapy with abrupt improvement in glycaemic control may be associated with temporary worsening of diabetic retinopathy, while long-term improved glycaemic control decreases the risk of progression of diabetic retinopathy.
Tabulated list of adverse reactions
Adverse reactions listed below are based on clinical trial data and classified according to MedDRA frequency and System Organ Class. Frequency categories are defined according to the following convention: Very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000); not known (cannot be estimated from the available data).
Immune system disorders
Uncommon – Urticaria, rash, eruptions
Very rare – Anaphylactic reactions*
Metabolism and nutrition disorders
Very common – Hypoglycaemia*
Nervous system disorders
Rare – Peripheral neuropathy (painful neuropathy)
Eye disorders
Uncommon – Refraction disorders
Uncommon – Diabetic retinopathy
Skin and subcutaneous tissue disorders
Uncommon – Lipodystrophy*
General disorders and administration site conditions
Uncommon – Injection site reactions
Uncommon – Oedema
* see section 4.8, Description of selected adverse reactions.
Description of selected adverse reactions
Anaphylactic reactions:
The occurrence of generalised hypersensitivity reactions (including generalised skin rash, itching, sweating, gastrointestinal upset, angioneurotic oedema, difficulties in breathing, palpitation and reduction in blood pressure) is very rare but can potentially be life threatening.
Hypoglycaemia:
The most frequently reported adverse reaction is hypoglycaemia. It may occur if the insulin dose is too high in relation to the insulin requirement. Severe hypoglycaemia may lead to unconsciousness and/or convulsions and may result in temporary or permanent impairment of brain function or even death. The symptoms of hypoglycaemia usually occur suddenly. They may include cold sweats, cool pale skin, fatigue, nervousness or tremor, anxiousness, unusual tiredness or weakness, confusion, difficulty in concentration, drowsiness, excessive hunger, vision changes, headache, nausea and palpitation.
In clinical trials, the frequency of hypoglycaemia varied with patient population, dose regimens and level of glycaemic control. During clinical trials the overall rates of hypoglycaemia did not differ between patients treated with insulin aspart compared to human insulin.
Lipodystrophy:
Lipodystrophy (including lipohypertrophy, lipoatrophy) may occur at the injection site. Continuous rotation of the injection site within the particular injection area reduces the risk of developing these reactions.
Paediatric population
Based on post-marketing sources and clinical trials, the frequency, type and severity of adverse reactions observed in the paediatric population do not indicate any differences to the broader experience in the general population.
Other special populations
Based on post-marketing sources and clinical trials, the frequency, type and severity of adverse reactions observed in the elderly patients and in patients with renal or hepatic impairment do not indicate any differences to the broader experience in the general population.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via:
Ireland
HPRA Pharmacovigilance
Earlsfort Terrace
IRL - Dublin 2
Tel: +353 1 6764971
Fax: +353 1 6762517
Website: www.hpra.ie
e-mail: medsafety@hpra.ie
Malta
ADR Reporting
Website: www.medicinesauthority.gov.mt/adrportal
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple store
4.9 Overdose
A specific overdose for insulin cannot be defined, however, hypoglycaemia may develop over sequential stages if too high doses relative to the patient's requirement are administered:
• Mild hypoglycaemic episodes can be treated by oral administration of glucose or sugary products. It is therefore recommended that the diabetic patient always carries sugar-containing products.
• Severe hypoglycaemic episodes, where the patient has become unconscious, can be treated with glucagon (0.5 to 1 mg) given intramuscularly or subcutaneously by a trained person, or with glucose given intravenously by physicians or other healthcare staff. Glucose must be given intravenously, if the patient does not respond to glucagon within 10 to 15 minutes. Upon regaining consciousness, administration of oral carbohydrates is recommended for the patient in order to prevent a relapse.
5. Pharmacological properties
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Drugs used in diabetes. Insulins and analogues for injection, fast-acting. ATC code: A10AB05.
Mechanism of action and pharmacodynamic effects
The blood glucose lowering effect of insulin aspart is due to the facilitated uptake of glucose following binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose output from the liver.
NovoRapid produces a more rapid onset of action compared to soluble human insulin, together with a lower glucose concentration, as assessed within the first four hours after a meal. NovoRapid has a shorter duration of action compared to soluble human insulin after subcutaneous injection
Fig. I. Blood glucose concentrations following a single pre-meal dose of NovoRapid injected immediately before a meal (solid curve) or soluble human insulin administered 30 minutes before a meal (hatched curve) in patients with type 1 diabetes mellitus.
When NovoRapid is injected subcutaneously, the onset of action will occur within 10 to 20 minutes of injection. The maximum effect is exerted between 1 and 3 hours after injection. The duration of action is 3 to 5 hours.
Clinical efficacy
Clinical trials in patients with type 1 diabetes have demonstrated a lower postprandial blood glucose with NovoRapid compared to soluble human insulin (Fig. I). In two long-term open label trials in patients with type 1 diabetes comprising 1070 and 884 patients, respectively, NovoRapid reduced glycated haemoglobin by 0.12 [95% C.I. 0.03; 0.22] percentage points and by 0.15 [95% C.I. 0.05; 0.26] percentage points compared to human insulin; a difference of limited clinical significance.
Clinical trials in patients with type 1 diabetes have demonstrated a reduced risk of nocturnal hypoglycaemia with insulin aspart compared with soluble human insulin. The risk of daytime hypoglycaemia was not significantly increased.
Insulin aspart is equipotent to soluble human insulin on a molar basis.
Special populations
Elderly (≥ 65 years old)
A randomised, double-blind cross-over PK/PD trial comparing insulin aspart with soluble human insulin was performed in elderly patients with type 2 diabetes (19 patients aged 65–83 years, mean age 70 years). The relative differences in the pharmacodynamic properties (GIRmax,AUCGIR, 0–120 min) between insulin aspart and soluble human insulin in the elderly were similar to those seen in healthy subjects and in younger patients with diabetes.
Paediatric population
A clinical trial comparing preprandial soluble human insulin with postprandial insulin aspart was performed in small children (20 patients aged 2 to less than 6 years, studied for 12 weeks, among those four were younger than 4 years old) and a single dose PK/PD trial was performed in children (6–12 years) and adolescents (13–17 years). The pharmacodynamic profile of insulin aspart in children was similar to that seen in adults.
The efficacy and safety of NovoRapid given as bolus insulin in combination with either insulin detemir or insulin degludec as basal insulin has been studied for up to 12 months, in two randomised controlled clinical trials in adolescents and children aged 1 to less than 18 years (n=712). The trials included 167 children aged 1–5 years, 260 aged 6–11 and 285 aged 12–17. The observed improvements in HbA1c and the safety profiles were comparable between all age groups.
Pregnancy
A clinical trial comparing safety and efficacy of insulin aspart vs. human insulin in the treatment of pregnant women with type 1 diabetes (322 exposed pregnancies (insulin aspart: 157; human insulin: 165)) did not indicate any adverse effect of insulin aspart on pregnancy or on the health of the foetus/newborn.
In addition the data from a clinical trial including 27 women with gestational diabetes randomised to treatment with insulin aspart vs. human insulin (insulin aspart: 14; human insulin: 13) showed similar safety profiles between treatments.
5.2 Pharmacokinetic properties
Absorption, distribution and elimination
In NovoRapid substitution of amino acid proline with aspartic acid at position B28 reduces the tendency to form hexamers as observed with soluble human insulin. NovoRapid is therefore more rapidly absorbed from the subcutaneous layer compared to soluble human insulin.
The time to maximum concentration is, on average, half of that for soluble human insulin. A mean maximum plasma concentration of 492±256 pmol/l was reached 40 (interquartile range: 30–40) minutes after a subcutaneous dose of 0.15 unit/kg bodyweight in type 1 diabetic patients. The insulin concentrations returned to baseline about 4 to 6 hours after dose. The absorption rate was somewhat slower in type 2 diabetic patients, resulting in a lower Cmax (352±240 pmol/l) and later tmax (60 (interquartile range: 50–90) minutes). The intra-individual variability in time to maximum concentration is significantly less for NovoRapid than for soluble human insulin, whereas the intra-individual variability in Cmax for NovoRapid is larger.
Special populations
Elderly (≥ 65 years old)
The relative differences in pharmacokinetic properties between insulin aspart and soluble human insulin in elderly patients (65–83 years, mean age 70 years) with type 2 diabetes were similar to those observed in healthy subjects and in younger patients with diabetes. A decreased absorption rate was observed in elderly patients, resulting in a later tmax (82 (interquartile range: 60–120) minutes), whereas Cmax was similar to that observed in younger patients with type 2 diabetes and slightly lower than in patients with type 1 diabetes.
Hepatic impairment
A single dose pharmacokinetic study of insulin aspart was performed in 24 subjects with hepatic function ranging from normal to severely impaired. In patients with hepatic impairment, absorption rate was decreased and more variable, resulting in delayed tmax from about 50 min in subjects with normal hepatic function to about 85 min in patients with moderate and severe hepatic impairment. AUC, Cmax and CL/F were similar in patients with reduced hepatic function compared with subjects with normal hepatic function.
Renal impairment
A single dose pharmacokinetic study of insulin aspart in 18 subjects with renal function ranging from normal to severely impaired was performed. No apparent effect of creatinine clearance values on AUC, Cmax, CL/F and tmax of insulin aspart was found. Data were limited in patients with moderate and severe renal impairment. Patients with renal failure necessitating dialysis treatment were not investigated.
Paediatric population
The pharmacokinetic and pharmacodynamic properties of NovoRapid were investigated in children (6–12 years) and adolescents (13–17 years) with type 1 diabetes. Insulin aspart was rapidly absorbed in both age groups, with similar tmax as in adults. However, Cmax differed between the age groups, stressing the importance of the individual titration of NovoRapid.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and toxicity to reproduction and development.
In in vitro tests, including binding to insulin and IGF-1 receptor sites and effects on cell growth, insulin aspart behaved in a manner that closely resembled human insulin. Studies also demonstrate that the dissociation of binding to the insulin receptor of insulin aspart is equivalent to human insulin.
6. Pharmaceutical particulars
6.1 List of excipients
Glycerol
Phenol
Metacresol
Zinc chloride
Disodium phosphate dihydrate
Sodium chloride
Hydrochloric acid (for pH adjustment)
Sodium hydroxide (for pH adjustment)
Water for injections
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: