- Anti-hestaminic & Respiratory Drugs (20)
- Anti-inflammatory Drugs (192) +-
- Baby & Mom (1305) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (52)
- Beauty (2855) +-
- Beauty > Skin Care > whitening (273)
- Chemotherapy & Immune Response (880) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (11)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (288)
- CIRCULATORY DISTURBANCE AGENTS (23)
- Diet & Fitness Products (278) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (192)
- HEMATOLOGY (39)
-
Medical Supplies (504)
+-
- Chemicals & Disinfectants (19)
- Dental Supplies (32)
- Devices & Instruments (10)
- Diabetic Supplies (121)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- KIDNEY UNIT SUPPLIES (21)
- Lab Supplies (3)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (2)
- Sanitary (5)
- Sterilization Supplies (0)
- Surgical Sutures (4)
- Syringes (3)
-
Medicines & Health (2584)
+-
- Allergy & Sinus (94)
- Children's Health Care (54)
- Cough, Cold & Flu (275)
- Digestive Health & Nausea (225)
- Ear, Nose & Throat Care (177)
- Eye Care (117)
- Feminine Care (321)
- Foot Care (4)
- Orthopaedic Appliances (0)
- Pain Relief & Management (237)
- Pill Organizer (2)
- Skin Treatments (773)
- Sleep & Snoring Aids (2)
- Support & Braces (6)
- Medicines & health > Gout releif (42)
- Natural & Organic Products (81) +-
- OTC > Analgesics > Anti-inflammatory Drugs (44)
-
Personal Care (3155)
+-
- Bath & Body (256)
- Deodorant & Anti-perspirants (180)
- Ear, Nose & Throat Care (173)
- Eye Care (123)
- Feminine Care (370)
- Foot Care (12)
- Hair Care (448)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (22)
- Massage & Relaxation (17)
- Natural & Organic Personal Care (7)
- Oral Care (89)
- Pregnancy & Fertility (64)
- Shaving & Grooming (65)
- Sun Care (77)
-
Prescription Drugs (2862)
+-
- Analgesics (181)
- Cardiovascular System (374)
- Drugs Affecting Musculoskeletal System (65)
- Drugs Used In Infections (56)
- Ear & Nose Drugs (2)
- Endocrine System (176)
- Gastrointestinal Tract (242)
- Gastrointestinal Tract > Hepatology > Liver treatment (61)
- GYNECOLOGY (2)
- Miscellaneous (11)
- NEPHROLOGY > URINARY SYSTEM > RENAL DISORDERS > URINARY TRACT DISORDERS (46)
- NEUROLOGY (223)
- Nutrients & Blood Electrolytes (2)
- Respiratory System (152)
- SKIN > NAILS > HAIR > TOPICAL PREPARATIONS (63)
- Vaccines (1)
- Prescription drugs > Cardiovascular system > Anti-hypertension drugs (242)
- Sexual Wellness (296) +-
- Vitamins & Minerals Supplements (1192) +-
Ex Tax: 80EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

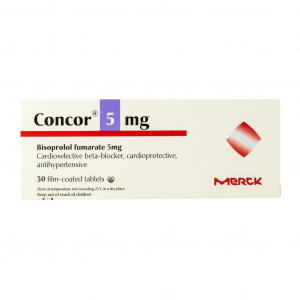
Concor ® 10 plus 10 / 25 mg ( Bisoprolol fumarate / Hydrochlorothiazide ) 30 tablets
Product information (abbreviated prescribing information)
Products:
Concor 5 plus, Concor 10 plus, film-coated tablets for oral use containing 5 mg bisoprolol
fumarate/12.5 mg hydrochlorothiazide, 10 mg bisoprolol fumarate/25 mg hydrochlorothiazide,
respectively. Different brand names are used for the products in some countries. Bisoprolol is
a beta1-selective adrenoceptor blocking agent, belonging to the pharmacotherapeutic group
of selective beta-blocking agents. Hydrochlorothiazide (HCTZ) is thiazide diuretic.
Indication:
Treatment of essential hypertension. The fixed dose combinations (Concor 5 plus, Concor 10
plus) are indicated in patients whose blood pressure is not adequately controlled on
bisoprolol fumarate or HCTZ alone.
Posology:
The fixed dose combinations Concor 5 plus, Concor 10 plus may be administered in patients
whose blood pressure is not adequately controlled by equivalent doses of bisoprolol
fumarate or HCTZ alone. Individual dose titration with the components can be recommended.
When clinically appropriate, direct change from monotherapy to the fixed combination may
be considered. Special populations: In patients with mild-to-moderate impairment of kidney
function the elimination of the HCTZ component of Concor 5 plus, Concor 10 is reduced, so
that preference may be given to the lower dose form Concor 5 plus. Since there is no
experience with Concor plus in children, its use for children cannot be recommended. The
treatment with bisoprolol must not be stopped abruptly, since this might lead to a transitory
worsening of condition, especially in patients with ischemic heart disease.
Contraindications:
Hypersensitivity to bisoprolol, hydrochlorothiazide, other thiazides, sulphonamides or to any
of the excipients; acute heart failure or during episodes of heart failure decompensation
requiring intravenous inotropic therapy, cardiogenic shock, second or third degree AV block,
sick sinus syndrome, sinoatrial block, symptomatic bradycardia, severe bronchial asthma,
severe forms of peripheral arterial occlusive disease or severe forms of Raynaud’s syndrome,
untreated phaeochromocytoma, severe renal impairment (creatinine clearance 30 ml/min),
severe hepatic impairment, metabolic acidosis, refractory hypkalemia, severe hyponatremia,
hypercalcemia, gout.
Warnings and precautions for use:
Bisoprolol must be used with caution in: bronchospasm (bronchial asthma, obstructive
airways disease; concomitant bronchodilating therapy may be recommended);
accompanying heart failure; diabetes mellitus showing large fluctuations in blood glucose
values, symptoms of hypoglycemia can be masked; strict fasting; first degree AV block;
Prinzmetal’s angina; peripheral arterial occlusive disease; hypovolemia; impaired liver
function. Bisoprolol may increase both the sensitivity towards allergens and the severity of
anaphylactic reactions. This also applies to desensitisation therapy. Patients with psoriasis or
with a history of psoriasis should only be given beta-blockers (e.g. bisoprolol) after a careful
balancing of benefits and risks. Symptoms of thyrotoxicosis may be masked. In patients
undergoing general anesthesia, the anesthesist must be aware of beta-blockade. If it is
thought necessary to withdraw beta-blocker therapy before surgery, this should be done
gradually and completed about 48 hours before anesthesia. Although cardioselective (beta1)
beta-blockers may have less effect on lung function than non-selective beta-blockers, as with
all beta-blockers, these should be avoided in patients with obstructive airways diseases,
unless there are compelling clinical reasons for their use. Where such reasons exist, Concor
Plus may be used with caution. If photosensitivity reactions occur, it is recommended to
protect exposed areas from the sun or from artificial UVA light. In severe cases it may be
necessary to stop treatment. Long-term continuous administration of HCTZ may lead to fluid
and electrolyte disturbances, in particular to hypokalemia and hyponatremia, also to
hypomagnesemia and hypochloremia, and hypercalcemia. Hypokalemia facilitates the
development of severe arrhythmias, particularly torsade de pointes, which may be fatal.
During long-term therapy with Concor plus, monitoring of serum electrolytes (especially
potassium, sodium, calcium), creatinine and urea, the serum lipids (cholesterol and
trigylcerides), uric acid as well as blood glucose is recommended. In patients with
hyperuricemia the risk of gout may be increased. Hydrochlorothiazide can cause an
idiosyncratic reaction, resulting in acute transient myopia and acute angle-closure glaucoma.
Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur
within hours to weeks of drug initiation. Untreated acute angle-closure glaucoma can lead to
permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly
as possible.
Ability to drive and use machines:
In general Concor plus has no or negligible influence on the ability to drive and use machines.
However, depending on the individual patients response to treatment the ability to drive a
vehicle or to use machines may be impaired. This needs to be considered particularly at start
of treatment, upon change of medication, or in conjunction with alcohol.
Interactions:
Combinations not recommended: Lithium, calcium antagonists of the verapamil and diltiazem
type, centrally-acting antihypertensive drugs. Combinations to be used with caution: Calcium
antagonists of the dihydropyridine type, other antihypertensive agents (particular caution for
ACE inhibitors and angiotensin II antagonists) and other drugs with blood pressure lowering
potential, class I or III antiarrhythmic drugs, antiarrhythmic and nonantiarrhythmic agents that
may induce torsade de pointes, parasympathomimetic agents, topical beta-blockers (e.g. eye
drops), insulin and oral antidiabetic agents, anesthetic agents, digitalis glycosides, nonsteroidal anti-inflammatory drugs (NSAIDs), sympathomimetic agents, potassium-wasting
medicinal products, methyldopa, uric-acid-lowering agents, cholestyramine, colestipol.
Combinations to be considered: Mefloquine, corticosteroids.
Pregnancy and lactation:
Concor plus is not recommended during pregnancy and also not in breastfeeding women.
HCTZ can inhibit the milk production.
Adverse reactions:
Common events seen included: hyperglycemia, hyperuricemia, disturbances of fluid and
electrolyte balance (in particular hypokalemia and hyponatremia, also hypomagnesemia and
hypochloremia as well as hypercalcemia), dizziness, headache, feeling of coldness or
numbness in the extremities, gastrointestinal complaints such as nausea, vomiting, diarrhoea,
constipation; fatigue, increased triglyceride and cholesterol levels, glucosuria.
Uncommon events seen included: loss of appetite, depression, sleep disorders, bradycardia,
AV-conduction disturbances, worsening of pre-existing heart failure, orthostatic hypotension,
bronchospasm in patients with bronchial asthma or a history of obstructive airway disease,
abdominal complaints, pancreatitis, muscle weakness, muscle cramps, asthenia, increase in
amylase, reversible increase in serum creatinine and urea.
Rare events seen included: leucopenia, thrombocytopenia, nightmares, hallucinations,
reduced tear flow, visual disturbances, hearing disorders, syncope, allergic rhinitis, hepatitis,
jaundice, hypersensitivity reactions such as itching, flush, rash, photodermatitis, purpura,
urticaria, potency disorders, increase in liver enzymes (ALAT, ASAT).
Very rare events seen included: agranulocytosis, metabolic alkalosis, conjunctivitis, alopecia,
cutaneous lupus erythematosus; beta-blockers may provoke or worsen psoriasis or induce
psoriasis-like rash; chest pain.
Not known events seen included: Interstitial lung disease.
Marketing Authorisation Holder:
Merck KGaA, Frankfurter Str. 250, 64293 Darmstadt, Germany; www.merckgroup.com
Date of product information preparation:
June 2016
Please always refer to the full prescribing information applicable in your country as this may
vary
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: