- Anti-hestaminic & Respiratory Drugs (14)
- Anti-inflammatory Drugs (130)
- Baby & Mom (1115) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (47)
- Beauty (2387) +-
- Beauty > Skin Care > whitening (230)
- Chemotherapy & Immune Response (253) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (2)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (82)
- CIRCULATORY DISTURBANCE AGENTS (15)
- CORTECOSTEROIDS (3)
- Diet & Fitness Products (186) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (138)
- Drugs affecting CNS >Anti- epileptic (46)
- LIVER SUPPORT>MULTIVITAMINS (22)
-
Medical Supplies (445)
+-
- Chemicals & Disinfectants (18)
- Dental Supplies (31)
- Devices & Instruments (7)
- Diabetic Supplies (91)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- Kindney Unit Supplies (13)
- Lab Supplies (1)
- Miscellaneous (22)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (4)
- Sanitary (5)
- Sterilization Supplies (0)
- Surgical Sutures (4)
- Syringes (2)
-
Medicines & Health (2163)
+-
- Allergy & Sinus (84)
- Children's Health Care (46)
- Cough, Cold & Flu (257)
- Digestive Health & Nausea (178)
- Ear, Nose & Throat Care (148)
- Eye Care (102)
- Feminine Care (283)
- Foot Care (3)
- Orthopaedic Appliances (0)
- Pain Relief & Management (180)
- Pill Organizer (0)
- Skin Treatments (633)
- Sleep & Snoring Aids (0)
- Support & Braces (7)
- Medicines & health > Gout releif (34)
- Natural & Organic Products (58) +-
- OTC > Analgesics > Anti-inflammatory Drugs (32)
-
Personal Care (2686)
+-
- Bath & Body (214)
- Deodorant & Anti-perspirants (159)
- Ear, Nose & Throat Care (141)
- Eye Care (108)
- Feminine Care (317)
- Foot Care (10)
- Hair Care (346)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (19)
- Massage & Relaxation (14)
- Natural & Organic Personal Care (6)
- Oral Care (76)
- Pregnancy & Fertility (51)
- Shaving & Grooming (55)
- Sun Care (52)
- Prescribtion drugs > cardiovascular system > Hypertention drugs (201)
-
Prescription Drugs (2143)
+-
- Analgesics (149)
- Cardiovascular System (269)
- Drugs Affecting CNS (155)
- Drugs Affecting Musculoskeletal System (46)
- Drugs Used In Infections (28)
- Ear & Nose Drugs (2)
- Endocrine System (124)
- Gastrointestinal Tract (178)
- Gastrointestinal Tract (165)
- Miscellaneous (2)
- Nutrients & Blood Electrolytes (0)
- Obstetric & Gynaecology Disorders (1)
- Respiratory System (96)
- Topical Preparations (21)
- Urinary Tract Disorders (12)
- Vaccines (0)
- Prescription Drugs > Cardiovascular System > Anti-hypertensive drugs > Duiretics > Loop duiretics (41)
- prescription drugs > cardiovascular system >Anti-hypertensive drugs > duiretics > Aldosterone antagonist duiretics (62)
- prescription drugs > cardiovascular system >Anti-hypertensive drugs > duiretics > duiretic combinations (59)
- prescription drugs > cardiovascular system >Anti-hypertensive drugs > duiretics > loop duiretics (59)
- Prescription Drugs > Gastrointestinal Tract > Liver treatment (46)
- Sexual Wellness (163) +-
- strong anti-emetic & adjuvent used with anti-neoplastic (1)
- Vitamins & Minerals Supplements (392)
- Vitamins & Supplements> folic acid (1597) +-
Ex Tax: 64EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

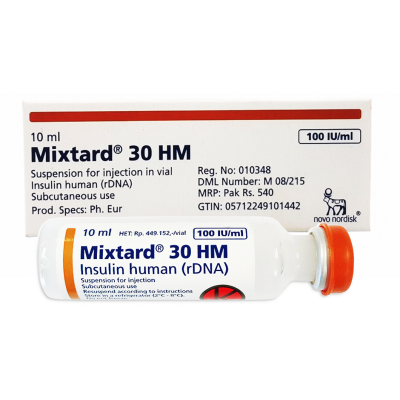
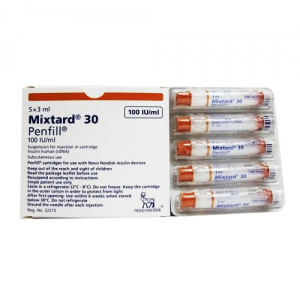
Mixtard ® 30 100 IU / ml ( Insulin human ( rDNA ) + Insulin Protophane Protamine ) 10 ml vial
Package Leaflet: Information for the user
Mixtard® 30 100 IU/ml suspension for injection in vial
Protaphane® 100 IU/ml suspension for injection in vial
Insulin human (rDNA)
Read all of this leaflet carefully before you start using your insulin
– Keep this leaflet. You may need to read it again.
– If you have any further questions, ask your doctor, nurse or your pharmacist.
– This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms
are the same as yours.
– If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your
doctor, nurse or your pharmacist.
1. WHAT MIXTARD® 30 AND PROTAPHANE® ARE AND WHAT THEY ARE USED
FOR
Mixtard® 30 and Protaphane® are human insulins used to treat diabetes. Diabetes mellitus is a disease where your
body does not produce enough insulin to control the level of your blood sugar. Mixtard® 30 is a mixture of fast-acting
insulin and long-acting insulin. This means that it will start to lower your blood sugar about ½ hour after you take it,
and the effect will last for approximately 24 hours. Protaphane® is a long-acting insulin. This means that it will start to
lower your blood sugar about 1½ hour after you take it, and the effect will last for approximately 24 hours. Protaphane®
is often given in combination with fast-acting insulin products.
2. BEFORE YOU USE MIXTARD® 30 OR PROTAPHANE®
Do not use Mixtard® 30 or Protaphane®:
► In insulin infusion pumps.
► If you are allergic (hypersensitive) to human insulin or any of the other ingredients in Mixtard® 30 or
Protaphane® (see 7. FURTHER INFORMATION).
► If you suspect hypoglycamia (low blood sugar) is starting (see 4. WHAT TO DO IN AN EMERGENCY).
► If the protective cap is loose or missing. Each vial has a protective, tamper-proof plastic cap. If it is not in
perfect condition when you get the vial, return the vial to your supplier.
► If it has not been stored correctly or been frozen (see 6. HOW TO STORE MIXTARD® 30 OR
PROTAPHANE®).
► If the resuspended insulin does not appear uniformly white and cloudy.
Before using Mixtard® 30 or Protaphane®
► Check the label to make sure it is the right type of insulin.
► Remove the protective cap.
Take special care with Mixtard® 30 or Protaphane®:
► If you have trouble with your kidneys or liver, or with your adrenal, pituitary or thyroid glands.
► If you drink alcohol, watch for signs of a hypo and never drink alcohol on an empty stomach.
If you exercise more than usual or if you want to change your usual diet, as this may affect your blood sugar
level.
► If you are ill, carry on taking your insulin and consult your doctor.
► If you are going abroad: travelling over time zones may affect your insulin needs and the timing of your
injections. Consult your doctor if you are planning such travelling.
Using other medicines
Some medicines affect the way glucose works in your body and this may influence your insulin dose. Listed below are
the most common medicines which may affect your insulin treatment. Tell to your doctor, nurse or pharmacist if you
are taking or have recently taken any other medicines, including medicines obtained without a prescription. In
particular, you should tell your doctor if you are using any medicine mentioned below that may affect your blood sugar
level.
Your need for insulin may change if you also take other medicines for treatment of diabetes; monoamine oxidase
inhibitors (MAOI); beta-blockers; ACE-inhibitors; acetylsalicylic acid; anabolic steroids; sulphonamides; oral
contraceptives; thiazides; glucocorticoids; thyroid hormone therapy; sympathomimetics; growth hormone; danazol;
octreotide or lanreotide.
Thiazolidinediones (class of oral antidiabetic medicines used for the treatment of type 2 diabetes mellitus)
Some patients with long-standing type 2 diabetes mellitus and heart disease or previous stroke who are treated with
thiazolidinediones in combination with insulin may develop heart failure. Inform your doctor as soon as possible if you
experience signs of heart failure such as unusual shortness of breath or rapid increase in weight or localised swelling
(oedema).
Pregnancy and breast-feeding
If you are pregnant, planning a pregnancy or breast-feeding, please contact your doctor for advice.
Driving and using machines
If you drive or use tools or machines, watch out for signs of a hypo. Your ability to concentrate or to react will be less
during a hypo. Never drive or use machinery if you feel a hypo coming on. Discuss with your doctor whether you can
drive or use machines at all, if you have a lot of hypos or if you find it hard to recognise hypos.
3. HOW TO USE MIXTARD® 30 OR PROTAPHANE®
Talk about your insulin needs with your doctor and nurse.
If your doctor has switched you from one type or brand of insulin to another, your dose may have to be adjusted by your
doctor.
Eat a meal or snack containing carbohydrates within 30 minutes of the injection.
It is recommended that you measure your blood sugar regularly.
How to use this insulin
Mixtard® 30 or Protaphane® are for injection under the skin (subcutaneously). Never inject your insulin directly
into a vein or muscle. Always vary the sites you inject within the same region, to reduce the risk of developing lumps or
skin pitting (see 5. POSSIBLE SIDE EFFECTS). The best places to give yourself an injection are: the front of your
waist (abdomen); your buttocks; the front of your thighs or upper arms. Your insulin will work more quickly if you
inject it around the waist.
How to inject Mixtard® 30/How to inject Protaphane® on its own or to mix with fast-acting insulin ► Make sure you have the correct syringe with the corresponding unit scale for insulin injections. ► Draw air into the syringe, in the same amount as the dose of insulin you need. ► Follow the instructions given by your healthcare professional. ► Just before injecting this insulin, roll the vial between your hands until the liquid is uniformly white and cloudy. Resuspending is easier if the insulin has reached room temperature. ► Inject the insulin under the skin. Use the injection technique advised by your doctor or nurse. ► Keep the needle under your skin for at least 6 seconds to make sure that the full dose has been delivered.
4. WHAT TO DO IN AN EMERGENCY If you get a hypo A hypo means your blood sugar level is too low. The warning signs of a hypo may come on suddenly and can include: cold sweat; cool pale skin; headache; rapid heart beat; feeling sick; feeling very hungry; temporary changes in vision; drowsiness; unusual tiredness and weakness; nervousness or tremor; feeling anxious; feeling confused; difficulty in concentrating. If you get any of these signs, eat glucose tablets or a high sugar snack (sweets, biscuits, fruit juice), then rest. Do not take any insulin if you feel a hypo coming on. Carry glucose tablets, sweets, biscuits or fruit juice with you, just in case. Tell your relatives, friends and close colleagues that if you pass out (become unconscious), they must turn you on your side and seek medical advice straight away. They must not give you any food or drink as it could choke you. ► If severe hypoglycaemia is not treated, it can cause brain damage (temporary or permanent) and even death. ► If you have a hypo that makes you pass out, or a lot of hypos, talk to your doctor. The amount or timing of insulin, food or exercise may need to be adjusted. Using glucagon You may recover more quickly from unconsciousness with an injection of the hormone glucagon by someone who knows how to use it. If you are given glucagon, you will need glucose or a sugary snack as soon as you are conscious. If you do not respond to glucagon treatment, you will have to be treated in a hospital. Seek medical advice after an injection of glucagon; you need to find the reason for your hypo to avoid getting more. Causes of a hypo You get a hypo if your blood sugar gets too low. This might happen: • If you take too much insulin. • If you eat too little or miss a meal. • If you exercise more than usual. If your blood sugar gets too high Your blood sugar may get too high (this is called hyperglycaemia). The warning signs appear gradually. They include: increased urination; feeling thirsty; losing your appetite; feeling sick (nausea or vomiting); feeling drowsy or tired; flushed, dry skin; dry mouth and a fruity (acetone) smell of the breath.
If you get any of these signs, test your blood sugar level and test your urine for ketones if you can. Then seek medical
advice straight away.
These may be signs of a very serious condition called diabetic ketoacidosis. If you do not treat it, this could lead to
diabetic coma and eventually death.
Causes of hyperglycaemia
• Having forgotten to take your insulin
• Repeatedly taking less insulin than you need
• An infection or a fever
• Eating more than usual
• Less exercise than usual
5. POSSIBLE SIDE EFFECTS
Like all medicines, Mixtard® 30 and Protaphane® can cause side effects, although not everybody gets them.
Side effects reported very commonly (in more than 1 patient in 10)
Low blood sugar (hypoglycaemia). See the advice in 4. WHAT TO DO IN AN EMERGENCY.
Side effects reported uncommonly (in less than 1 patient in 100)
Changes at the injection site (lipodystrophy). . The fatty tissue under the skin at the injection site may shrink
(lipoatrophy) or thicken (lipohypertrophy). Changing the site with each injection may help to reduce the risk of
developing such skin changes. If you notice your skin pitting or thickening at the injection site, tell your doctor or nurse.
These reactions can become more severe, or they may change the absorption of your insulin if you inject in such a site.
Signs of allergy. Reactions (redness, swelling, itching) at the injection site may occur (local allergic reactions). These
usually disappear after a few weeks of taking your insulin. If they do not disappear, see your doctor.
Seek medical advice immediately:
• If signs of allergy spread to other parts of the body, or
• If you suddenly feel unwell, and you: start sweating; start being sick (vomiting); have difficulty in breathing;
have a rapid heart beat, feel dizzy.
Diabetic retinopathy (eye disease related to diabetes which can lead to loss of vision). If you have diabetic retinopathy
and your blood sugar level improves very fast, the retinopathy may get worse. Ask your doctor about this.
Swollen joints. When you start taking insulin, water retention may cause swelling around your ankles and other joints.
Normally this soon disappears.
(Mixtard® 30) Painful neuropathy (pain due to nerve damage). If your blood sugar level improves very fast, you may
get nerve related pain, this is called acute painful neuropathy and is usually transient.
Side effects reported very rarely (in less than 1 patient in 10,000)
Vision problems. When you first start your insulin treatment, it may disturb your vision, but the disturbance is usually
temporary.
(Protaphane®) Painful neuropathy (pain due to nerve damage). If your blood sugar level improves very fast, you may
get nerve related pain, this is called acute painful neuropathy and is usually transient.
Serious allergic reaction to Mixtard® 30 or Protaphane® or one of their ingredients (called a systemic allergic
reaction). See also the warning in 4. WHAT TO DO IN AN EMERGENCY.
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor,
nurse or pharmacist.
6. HOW TO STORE MIXTARD® 30 OR PROTAPHANE®
Keep out of the reach and sight of children.
Do not use Mixtard® 30 or Protaphane® after the expiry date which is stated on the label and the carton after ‘EXP.’ The
expiry date refers to the last day of that month.
Mixtard® vial or Protaphane® vial that is not being used is to be stored in the refrigerator at 2°C - 8°C, away from
the cooling element. Do not freeze.
Mixtard® vial or Protaphane® vial that is being used or carried as a spare is not to be kept in a refrigerator. After
removing the vial from the refrigerator, it is recommended to let it reach room temperature before resuspending the
insulin as instructed for the first time use. See 3. HOW TO USE MIXTARD® 30 OR PROTAPHANE®.
You can carry it with you and keep it at room temperature (below 25ºC) for up to 6 weeks.
Always keep the vial in the outer carton when you are not using it in order to protect it from light.
Mixtard® 30 and Protaphane® must be protected from excessive heat and light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of
medicines no longer required. These measures will help to protect the environment.
7. FURTHER INFORMATION
What Mixtard® 30 contains
- The active substance is insulin human made by recombinant biotechnology as 30% as soluble insulin and 70% as
isophane insulin, 1 ml contains 100 IU of insulin human. 1 vial contains 10 ml equivalent to 1,000 IU.
- The other ingredients are zinc chloride, glycerol, metacresol, phenol, disodium phosphate dihydrate, sodium
hydroxide, hydrochloric acid, protamine sulphate and water for injections.
What Protaphane® contains
- The active substance is insulin human made by recombinant biotechnology. Protaphane® is an isophane insulin
suspension (NPH). 1 ml contains 100 IU of insulin human. 1 vial contains 10 ml equivalent to 1,000 IU.
- The other ingredients are zinc chloride, glycerol, metacresol, phenol, disodium phosphate dihydrate, sodium
hydroxide, hydrochloric acid, protamine sulphate and water for injections.
What Mixtard® or Protaphane® looks like and contents of the pack
The suspension for injection comes as a cloudy, white, aqueous suspension.
It is supplied in packs of 1 vial of 10 ml. Mixtard® and Protaphane® are trademarks owned by Novo Nordisk A/S. © 2012 Marketed by Novo Nordisk Pharmaceuticals Ltd. Auckland, New Zealand This leaflet was last approved in June 2012.
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: