- Anti-hestaminic & Respiratory Drugs (14)
- Anti-inflammatory Drugs (134)
- Baby & Mom (1112) +-
- Baby & Mom > Bath, skin & Hair > Skin Care > wibes (47)
- Beauty (2408) +-
- Beauty > Skin Care > whitening (232)
- Chemotherapy & Immune Response (288) +-
- Chemotherapy & Immune Response > ANTI-FUNGAL (2)
- Chemotherapy & Immune Response > Chemotherapeutic Agents > Hormone Antagonists >Enzyme Inhibitors (92)
- CIRCULATORY DISTURBANCE AGENTS (15)
- CORTECOSTEROIDS (6)
- Diet & Fitness Products (186) +-
- DRUG AFFECTING CENTRAL NERVOUS SYSTEM (138)
- Drugs affecting CNS >Anti- epileptic (46)
- LIVER SUPPORT>MULTIVITAMINS (22)
-
Medical Supplies (448)
+-
- Chemicals & Disinfectants (18)
- Dental Supplies (31)
- Devices & Instruments (8)
- Diabetic Supplies (91)
- General Medical Supplies (21)
- I.V & Medical Solution (0)
- Intensive Care Unit & Anesthesia Supplies (0)
- Kindney Unit Supplies (13)
- Lab Supplies (1)
- Miscellaneous (21)
- Neonatal Unit Supplies (0)
- Operation Room Supplies (4)
- Sanitary (5)
- Sterilization Supplies (0)
- Surgical Sutures (4)
- Syringes (2)
-
Medicines & Health (2175)
+-
- Allergy & Sinus (84)
- Children's Health Care (46)
- Cough, Cold & Flu (258)
- Digestive Health & Nausea (178)
- Ear, Nose & Throat Care (148)
- Eye Care (102)
- Feminine Care (285)
- Foot Care (3)
- Orthopaedic Appliances (0)
- Pain Relief & Management (180)
- Pill Organizer (0)
- Skin Treatments (639)
- Sleep & Snoring Aids (0)
- Support & Braces (7)
- Medicines & health > Gout releif (34)
- Natural & Organic Products (59) +-
- OTC > Analgesics > Anti-inflammatory Drugs (32)
-
Personal Care (2702)
+-
- Bath & Body (214)
- Deodorant & Anti-perspirants (159)
- Ear, Nose & Throat Care (142)
- Eye Care (108)
- Feminine Care (319)
- Foot Care (10)
- Hair Care (350)
- Home Tests & Monitorings (14)
- Incontinence (7)
- Lip Care (19)
- Massage & Relaxation (14)
- Natural & Organic Personal Care (6)
- Oral Care (76)
- Pregnancy & Fertility (52)
- Shaving & Grooming (55)
- Sun Care (52)
- Prescribtion drugs > cardiovascular system > Hypertention drugs (207)
-
Prescription Drugs (2186)
+-
- Analgesics (149)
- Cardiovascular System (278)
- Drugs Affecting CNS (155)
- Drugs Affecting Musculoskeletal System (46)
- Drugs Used In Infections (29)
- Ear & Nose Drugs (2)
- Endocrine System (124)
- Gastrointestinal Tract (179)
- Gastrointestinal Tract (166)
- Miscellaneous (2)
- Nutrients & Blood Electrolytes (0)
- Obstetric & Gynaecology Disorders (1)
- Respiratory System (100)
- Topical Preparations (21)
- Urinary Tract Disorders (12)
- Vaccines (0)
- Prescription Drugs > Cardiovascular System > Anti-hypertensive drugs > Duiretics > Loop duiretics (46)
- prescription drugs > cardiovascular system >Anti-hypertensive drugs > duiretics > Aldosterone antagonist duiretics (63)
- prescription drugs > cardiovascular system >Anti-hypertensive drugs > duiretics > duiretic combinations (60)
- prescription drugs > cardiovascular system >Anti-hypertensive drugs > duiretics > loop duiretics (60)
- Prescription Drugs > Gastrointestinal Tract > Liver treatment (47)
- Sexual Wellness (165) +-
- strong anti-emetic & adjuvent used with anti-neoplastic (1)
- Vitamins & Minerals Supplements (395)
- Vitamins & Supplements> folic acid (1606) +-
Ex Tax: 900EGP
Example
You can return the product within 14 days of purchase.
ReturnsYou can return the product within 14 days of purchase.

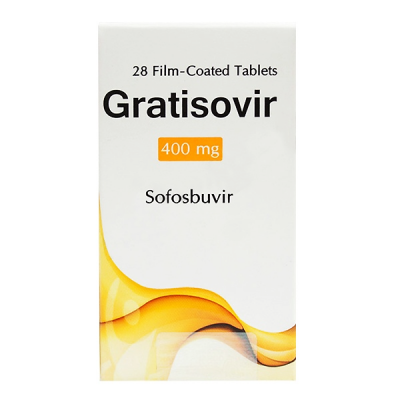
Sofosbuvir was a breakthrough new medication for the treatment of patients with chronic hepatitis C. Sofosbuvir has a number of ideal properties, including once daily dosing, no meal restrictions, few adverse effects, minimal drug-drug interactions, high genetic barrier to resistance, and relatively good safety and efficacy in patients with advanced liver disease. The use of sofosbuvir in combination with ribavirin was the first FDA-approved all oral therapy for hepatitis C. Of note, the activity against genotype 3 appears less than with genotype 2 and treatment of genotype 3 infection requires a longer all-oral course of treatment than with genotype 2. Sofosbuvir is primarily used now in fixed-dose combinations.
Sofosbuvir is generally well-tolerated. The most common adverse effects observed with sofosbuvir, when used in combination with ribavirin, have been fatigue and headache. Sofosbuvir is pregnancy category B. To report suspected adverse reactions, contact (1) Gilead Sciences, Inc. at 1-800-GILEAD-5 or (2) the FDA at 1-800-FDA-1088.
CLASS AND MECHANISM
Sofosbuvir is a nucleotide analog inhibitor of hepatitis C virus NS5B polymerase—the key enzyme mediating HCV RNA replication. Sofosbuvir is a prodrug and after ingestion it is rapidly converted to GS-331007, the predominant circulating drug that accounts for greater than 90% of the systemically active drug. The compound GS-331007 is efficiently taken up by hepatocytes, whereby cellular kinases convert GS-331007 to its pharmacologically active uridine analog 5’-triphosphate form (GS-461203). This triphosphate compound mimics the natural cellular uridine nucleotide and is incorporated by the HCV RNA polymerase into the elongating RNA primer strand, resulting in chain termination. The active form GS-461203 targets the NS5B catalytic site and acts as a non-obligate chain terminator. The active compound (GS-461203) does not inhibit host DNA polymerases, RNA polymerases, or mitochondrial RNA polymerase.
INDICATIONS
The following indications for sofosbuvir relate to patients with chronic hepatitis C virus infection.
Sofosbuvir is indicated for treatment of patients with chronic HCV
Genotype 1 or 4: sofosbuvir plus peginterferon-alfa plus ribavirin for 12 weeks
Genotype 2: sofosbuvir plus ribavirin for 12 weeks
Genotype 3: sofosbuvir plus ribavirin for 24 weeks
For the treatment of patients with HCV-HIV-1 coinfection: the recommendations are the same as listed above.
For patents with genotype 1 HCV who are not eligible to receive interferon: sofosbuvir plus ribavirin for 24 weeks can be considered.
For patients with HCV and hepatocellular carcinoma awaiting liver transplantation: sofosbuvir plus ribavirin for a duration of up to 48 weeks or until liver transplantation, whichever occurs first.
Simeprevir in Combination with Sofosbuvir for HCV Infection: for details see the Simeprevir Drug Summary page and Simeprevir Full Prescribing Information
Daclatasvir in Combination with Sofosbuvir for HCV Infection: for details see the Daclatasvir Drug Summary page and Daclatasvir Full Prescribing Information
CONTRAINDICATIONS
When sofosbuvir is used in combination with peginterferon alfa plus ribavirin, or with ribavirin alone, all contraindications that pertain to peginterferon alfa or ribavirin will apply to the combination regimens. The use of sofosbuvir with amiodarone is not recommended due to the risk of developing serious symptomatic bradycardia; this risk is pronounced in persons also taking a beta-blocker and for those with underlying cardiac comorbidities or advanced liver disease.
DOSING
Sofosbuvir is available as a 400 mg tablet. The recommended dose of sofosbuvir is 400 mg taken orally once daily, with or without food. The 400 mg dose of sofosbuvir should be used, regardless of the patient's genotype and prior hepatitis C treatment experience. No dose adjustment is needed for mild-to-moderate renal impairment or with mild, moderate, or severe hepatic impairment. The prescribing information does not make a recommendation for dosing in patients who have severe renal impairment (eGFR less than 30 ml/min/1.73m2) or end stage renal disease requiring dialysis. Sofosbuvir is also available as a fixed-dose combination pill (ledipasvir 90 mg and sofosbuvir 400 mg) taken once daily.
Write a review
Your Name:Your Review: Note: HTML is not translated!
Rating: Bad Good
Enter the code in the box below: